
Which of the following is not a saturated hydrocarbon?
(A) Cyclohexane
(B) Benzene
(C) Butane
(D) Pentane
Answer
233.4k+ views
Hint: Saturated means that the hydrocarbon (molecules containing only hydrogen and carbon) has only single bonds and that the hydrocarbon contains the maximum number of hydrogen atoms for each carbon atom. For example alkanes.
Complete step by step solution:
Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms.
Now, the term unsaturated (not saturated) means that more hydrogen atoms may be added to the hydrocarbon to make it saturated (i.e. consisting of all single bonds).
All the hydrocarbons containing “ane” suffix contain a single bond between the carbon atoms whereas those containing “ene”suffix contain double bonds between carbon atoms and those having “yne” suffix contain triple bonds between carbon atoms.
Therefore, among the given options, Benzene is not a saturated hydrocarbon due to the presence of double bonds. It is a molecule which is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Its structure is as shown:
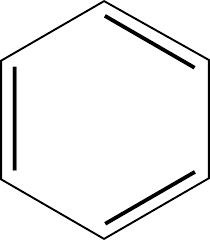
Hence, Option B is correct.
Note: Benzene is a clear, colorless, highly flammable and volatile liquid aromatic hydrocarbon with a gasoline like odor. It is used in many chemical industries to make plastics, resins, nylon and synthetic fibers. It is also used to make some types of lubricants, rubbers, dyes, detergents, drugs and pesticides.
Complete step by step solution:
Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms.
Now, the term unsaturated (not saturated) means that more hydrogen atoms may be added to the hydrocarbon to make it saturated (i.e. consisting of all single bonds).
All the hydrocarbons containing “ane” suffix contain a single bond between the carbon atoms whereas those containing “ene”suffix contain double bonds between carbon atoms and those having “yne” suffix contain triple bonds between carbon atoms.
Therefore, among the given options, Benzene is not a saturated hydrocarbon due to the presence of double bonds. It is a molecule which is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Its structure is as shown:

Hence, Option B is correct.
Note: Benzene is a clear, colorless, highly flammable and volatile liquid aromatic hydrocarbon with a gasoline like odor. It is used in many chemical industries to make plastics, resins, nylon and synthetic fibers. It is also used to make some types of lubricants, rubbers, dyes, detergents, drugs and pesticides.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




