
Which of the following is not a redox reaction ?
A . $CaC{O_3} \to CaO + C{O_2}$
B . ${O_2} + 2{H_2} \to 2{H_2}O$
C . $Na + {H_2}O \to NaOH + \dfrac{1}{2}{H_2}$
D . $MnC{l_3} \to MnC{l_2} + \dfrac{1}{2}C{l_2}$
Answer
532.9k+ views
Hint : In redox reaction one species is reduced and another is oxidized. We can find out the redox reaction if there has been a transfer of electrons means there should be change in the oxidation number of the reactants and the products. We will check one by one each reaction by calculating the oxidation number of all species.
Complete answer:
A . $CaC{O_3} \to CaO + C{O_2}$ is a decomposition reaction . Here in this reaction we can see that there is no change in the oxidation number of any species so it is not a redox reaction.
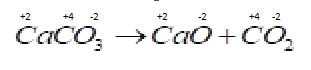
B . ${O_2} + 2{H_2} \to 2{H_2}O$ Now in this reaction oxidation number of different species are changing so the reduction and oxidation both process occurs in the same reaction so it is a redox reaction.
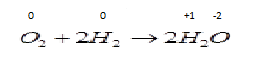
C . $Na + {H_2}O \to NaOH + \dfrac{1}{2}{H_2}$ ; in this reaction oxidation number of species changes so it is also a redox reaction.
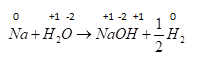
D . $MnC{l_3} \to MnC{l_2} + \dfrac{1}{2}C{l_2}$ Now let's check last reaction ,oxidation number of $Mn$ changes from $ + 3$ to $ + 2$ and $Cl$ changes from $ - 1$ to zero so it is also a redox reaction.
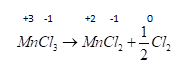
Hence only option A is not a redox reaction as there is no change in the oxidation number of its species.
Note : We have approached this problem by the concept that in a redox reaction reduction and oxidation both process occurs simultaneously and there is always change in the oxidation number of the reactant and product .In this problem we have found oxidation number of all four reaction , only in option A oxidation number of reactant and product species remains same so this is not a redox reaction.
Complete answer:
A . $CaC{O_3} \to CaO + C{O_2}$ is a decomposition reaction . Here in this reaction we can see that there is no change in the oxidation number of any species so it is not a redox reaction.
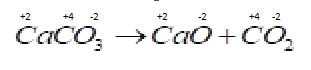
B . ${O_2} + 2{H_2} \to 2{H_2}O$ Now in this reaction oxidation number of different species are changing so the reduction and oxidation both process occurs in the same reaction so it is a redox reaction.
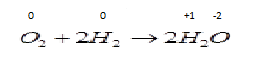
C . $Na + {H_2}O \to NaOH + \dfrac{1}{2}{H_2}$ ; in this reaction oxidation number of species changes so it is also a redox reaction.
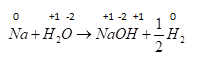
D . $MnC{l_3} \to MnC{l_2} + \dfrac{1}{2}C{l_2}$ Now let's check last reaction ,oxidation number of $Mn$ changes from $ + 3$ to $ + 2$ and $Cl$ changes from $ - 1$ to zero so it is also a redox reaction.
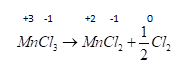
Hence only option A is not a redox reaction as there is no change in the oxidation number of its species.
Note : We have approached this problem by the concept that in a redox reaction reduction and oxidation both process occurs simultaneously and there is always change in the oxidation number of the reactant and product .In this problem we have found oxidation number of all four reaction , only in option A oxidation number of reactant and product species remains same so this is not a redox reaction.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Understanding Electromagnetic Waves and Their Importance

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

CBSE Notes Class 11 Chemistry Chapter 8 - Organic Chemistry Some Basic Principles And Techniques - 2025-26




