
Which of the following is not a postulate given by Rutherford?
A. The positively charged particles are present in the nucleus of atom
B. Size of nucleus is very small as compare to the size of atom
C. The whole mass of the atom is concentrated in the center of atom called nucleus
D. An atom consists of a positively charged sphere, with electrons set within the sphere
Answer
233.1k+ views
Hint: Rutherford conducted an experiment by bombarding a thin sheet of gold with -particles and then studied the trajectory of these particles after their interaction with the gold foil.
Complete step by step answer:
Ernest Rutherford, a British scientist conducted an experiment and based on the observations of this experiment, he proposed the atomic structure of the elements and further gave the Rutherford atomic model. Now, let’s see how Rutherford performed the experiment.
In his experiment, Rutherford directed high energy of $\alpha $ particles from a radioactive source at a thin sheet of gold. In order to study the deflection caused by the alpha particles, he placed a fluorescent zinc sulphate screen around the thin gold foil.
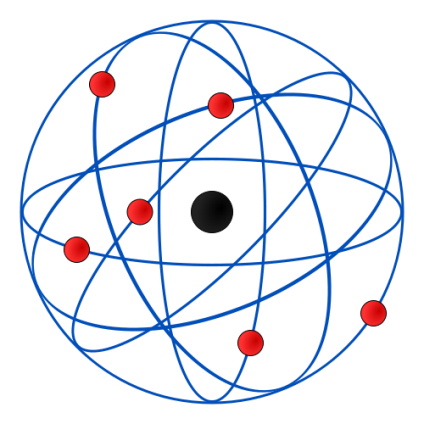
Now, after performing the experiment, he concluded that:
The nucleus is at the center and is positively charged and nearly all the mass of the nucleus resides in the nucleus. He also said that electrons revolve around the nucleus in a circular path. Also, the size of the nucleus is very less as compared to the size of the atom.
Therefore, he never concluded that the atom consists of a positively charged sphere, with electrons set within the sphere.
Hence, option D is correct.
Note: Although the model was based on the experimental observations, it further failed to explain certain things such as he did not say anything about the arrangement of electrons in an atom which made his theory incomplete. Moreover, the model could not explain the stability of an atom as well.
Complete step by step answer:
Ernest Rutherford, a British scientist conducted an experiment and based on the observations of this experiment, he proposed the atomic structure of the elements and further gave the Rutherford atomic model. Now, let’s see how Rutherford performed the experiment.
In his experiment, Rutherford directed high energy of $\alpha $ particles from a radioactive source at a thin sheet of gold. In order to study the deflection caused by the alpha particles, he placed a fluorescent zinc sulphate screen around the thin gold foil.

Now, after performing the experiment, he concluded that:
The nucleus is at the center and is positively charged and nearly all the mass of the nucleus resides in the nucleus. He also said that electrons revolve around the nucleus in a circular path. Also, the size of the nucleus is very less as compared to the size of the atom.
Therefore, he never concluded that the atom consists of a positively charged sphere, with electrons set within the sphere.
Hence, option D is correct.
Note: Although the model was based on the experimental observations, it further failed to explain certain things such as he did not say anything about the arrangement of electrons in an atom which made his theory incomplete. Moreover, the model could not explain the stability of an atom as well.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




