
Which of the following is not a natural polymer?
(A) Proteins
(B) Polysaccharides
(C) Cotton
(D) Phenol-formaldehyde
Answer
233.1k+ views
Hint: - The alpha amino acids are considered to be the building blocks of proteins.
- Polysaccharides are carbohydrates which upon hydrolysis give a large number of monosaccharides.
- Bakelite is a phenol-formaldehyde polymer.
Complete step by step answer:
Amino acids are those organic compounds which contain amino groups and carboxyl groups as functional groups. Depending upon the relative position of the amino group with respect to the carboxyl group, the amino acids are classified as alpha-, beta-, gamma-, delta- etc. amino acids.
The hydrolysis of proteins gives only the alpha amino acids. The term ‘amino acid’ is used to refer specifically to these alpha amino acids.
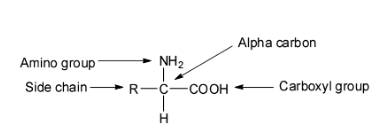
Thus, the proteins are biopolymers formed by the condensation of a large number of alpha amino acids joined together by peptide bonds having 3D structures. So, option A is wrong.
Polysaccharides are biopolymers formed by joining together the monosaccharides by glycosidic linkages. For example, cellulose is a polysaccharide.
Cellulose is a linear polymer of $\beta$ -D-glucose in which ${{\text{C}}_{\text{1}}}$ of one glucose unit is linked to ${{\text{C}}_4}$ of the other glucose unit through a $\beta$-glycosidic linkage. Hence, option B is not correct.
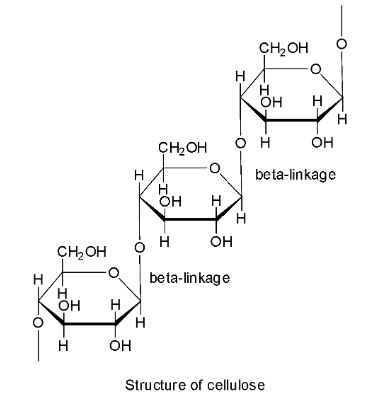
The chief component of cotton is cellulose. Cotton consists of about 95 percent cellulose and the rest are fats and waxes. Thus, cotton is also a natural polymer and so option C is wrong.
Bakelite is a synthetic polymer obtained by the condensation of phenol with formaldehyde in presence of an acid or a base catalyst. The initially formed ortho or para hydroxyl derivatives further react with phenol to give novolac which on further heating with formaldehyde gives bakelite by cross linking.
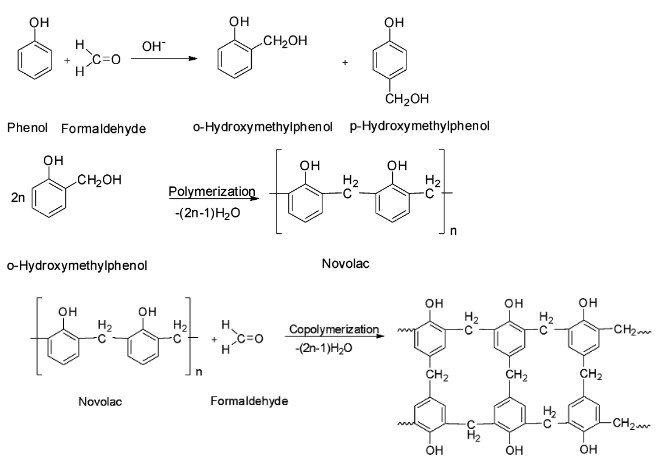
Thus, Bakelite is a synthetic polymer and hence option D is correct.
Note:
Bakelite is used in the manufacture of combs, fountain pen barrels and also used for making electrical goods.
The polysaccharide cellulose is considered as the chief structural material of cell walls of all plants. Due to its linear structure, cellulose is easily converted into fibres.
Cotton is most commonly used to make a number of textile products.
- Polysaccharides are carbohydrates which upon hydrolysis give a large number of monosaccharides.
- Bakelite is a phenol-formaldehyde polymer.
Complete step by step answer:
Amino acids are those organic compounds which contain amino groups and carboxyl groups as functional groups. Depending upon the relative position of the amino group with respect to the carboxyl group, the amino acids are classified as alpha-, beta-, gamma-, delta- etc. amino acids.
The hydrolysis of proteins gives only the alpha amino acids. The term ‘amino acid’ is used to refer specifically to these alpha amino acids.

Thus, the proteins are biopolymers formed by the condensation of a large number of alpha amino acids joined together by peptide bonds having 3D structures. So, option A is wrong.
Polysaccharides are biopolymers formed by joining together the monosaccharides by glycosidic linkages. For example, cellulose is a polysaccharide.
Cellulose is a linear polymer of $\beta$ -D-glucose in which ${{\text{C}}_{\text{1}}}$ of one glucose unit is linked to ${{\text{C}}_4}$ of the other glucose unit through a $\beta$-glycosidic linkage. Hence, option B is not correct.

The chief component of cotton is cellulose. Cotton consists of about 95 percent cellulose and the rest are fats and waxes. Thus, cotton is also a natural polymer and so option C is wrong.
Bakelite is a synthetic polymer obtained by the condensation of phenol with formaldehyde in presence of an acid or a base catalyst. The initially formed ortho or para hydroxyl derivatives further react with phenol to give novolac which on further heating with formaldehyde gives bakelite by cross linking.

Thus, Bakelite is a synthetic polymer and hence option D is correct.
Note:
Bakelite is used in the manufacture of combs, fountain pen barrels and also used for making electrical goods.
The polysaccharide cellulose is considered as the chief structural material of cell walls of all plants. Due to its linear structure, cellulose is easily converted into fibres.
Cotton is most commonly used to make a number of textile products.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




