
Which of the following is isomorphous with \[{\text{MgS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\] ?
(A) Green vitriol
(B) Blue vitriol
(C) Red vitriol
(D) Vitriol of Mars
Answer
233.1k+ views
Hint: - Two substances are said to be isomorphous if they have the same chemical formulation.
- Isomorphous crystals must possess atoms having corresponding chemical properties. The size of the corresponding atoms should also be similar.
Complete step by step answer:
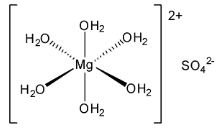
Epsom salt is actually magnesium sulfate in the most common form which is magnesium sulfate heptahydrate or \[{\text{MgS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\] . It loses one equivalent of water to form the hexahydrate which has octahedral geometry.
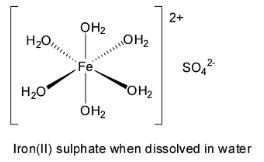
Green vitriol is another name of crystalline ferrous sulphate or iron (II) sulphate. The most commonly encountered form is the heptahydrate form. It has the chemical formula \[{\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\] . It is bluish – green in colour. It dissolves in water to give the hexahydrated form which has octahedral geometry.
Thus, green vitriol has the same chemical formulation as magnesium sulphate heptahydrate or Epsom salt. They also have similar shape and chemical properties. So, they are isomorphous and hence, option A is correct.
Blue vitriol is just another name for copper sulphate or copper (II) sulphate. The most commonly encountered form of copper sulphate is copper sulphate pentahydrate which is bright blue in colour. It has the chemical formula \[{\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}\] . Thus, it has a different chemical formulation than that of magnesium sulphate heptahydrate and so they are not isomorphous. So, option B is wrong.
Red vitriol is cobalt (II) sulphate heptahydrate having the chemical formula \[{\text{CoS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\]. The size of the cobalt atom is not similar to that of magnesium in Epsom salt and hence C is also wrong.
Vitriol of Mars is ferric sulphate or iron (III) sulphate having the chemical formula \[{\text{F}}{{\text{e}}_2}{\left( {{\text{S}}{{\text{O}}_{\text{4}}}} \right)_3}\] . Thus, it has a different chemical formulation than that of magnesium sulphate heptahydrate and so they are not isomorphous. So, option D is wrong.
Note:
- The main external use of Epsom salt is the formulation of bath salts to treat sore feet. It is also used as a saline laxative.
- Epsom salt also leads to the release of cholecystokinin and this in turn causes the increase of intestinal motility.
- Isomorphous crystals must possess atoms having corresponding chemical properties. The size of the corresponding atoms should also be similar.
Complete step by step answer:
Epsom salt is actually magnesium sulfate in the most common form which is magnesium sulfate heptahydrate or \[{\text{MgS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\] . It loses one equivalent of water to form the hexahydrate which has octahedral geometry.

Green vitriol is another name of crystalline ferrous sulphate or iron (II) sulphate. The most commonly encountered form is the heptahydrate form. It has the chemical formula \[{\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\] . It is bluish – green in colour. It dissolves in water to give the hexahydrated form which has octahedral geometry.

Thus, green vitriol has the same chemical formulation as magnesium sulphate heptahydrate or Epsom salt. They also have similar shape and chemical properties. So, they are isomorphous and hence, option A is correct.
Blue vitriol is just another name for copper sulphate or copper (II) sulphate. The most commonly encountered form of copper sulphate is copper sulphate pentahydrate which is bright blue in colour. It has the chemical formula \[{\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}\] . Thus, it has a different chemical formulation than that of magnesium sulphate heptahydrate and so they are not isomorphous. So, option B is wrong.
Red vitriol is cobalt (II) sulphate heptahydrate having the chemical formula \[{\text{CoS}}{{\text{O}}_{\text{4}}}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\]. The size of the cobalt atom is not similar to that of magnesium in Epsom salt and hence C is also wrong.
Vitriol of Mars is ferric sulphate or iron (III) sulphate having the chemical formula \[{\text{F}}{{\text{e}}_2}{\left( {{\text{S}}{{\text{O}}_{\text{4}}}} \right)_3}\] . Thus, it has a different chemical formulation than that of magnesium sulphate heptahydrate and so they are not isomorphous. So, option D is wrong.
Note:
- The main external use of Epsom salt is the formulation of bath salts to treat sore feet. It is also used as a saline laxative.
- Epsom salt also leads to the release of cholecystokinin and this in turn causes the increase of intestinal motility.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




