
Which of the following is an example of copolymer?
A.Buna-S
B.Teflon
C.PVC
D.Polypropylene
Answer
233.1k+ views
Hint: Copolymer is basically a polymer that is made up of two or more monomer species. Moreover, many commercially important polymers are copolymers. The polymerization of monomers into copolymers is called as copolymerization.
Complete step by step answer:
A polymer is a large molecule or a macromolecule which is further a combination of many subunits. They can be naturally found in plants and animals or are man-made. Basically, there are three types of polymers:
1.Natural
2.Synthetic
3.Semi-synthetic
Basically, when two different types of monomers are joined together in the same polymer chain, then the polymer formed is known as a co-polymer. Moreover, the process in which a polymer is formed from multiple species of monomers, is known as copolymerization and the copolymers obtained by copolymerization of two monomer species are called as biopolymers.
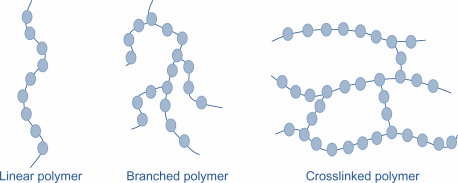
Now, co- polymers are classified as:
1.Linear copolymers (single chain)
2.Branched copolymers (polymeric chain)
3.Cross-linked polymer (composed of bifunctional and trifunctional monomers)
The structures are as shown:
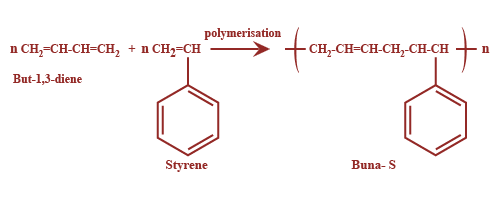
Now, among the given options Buna-S is a copolymer. It is made up of two monomers i.e. 1,3- butadiene and Styrene. It is also known as Styrene-Butadiene Rubber. It is used for making automobile tyres and rubber soles. The polymerization is as shown:
Hence, option A is correct.
Note: Teflon is made up of a chemical compound named polytetrafluoroethylene which is a synthetic fluoropolymer. This compound is used as the coating for non-stick pans as they are hydrophobic and have a high resistance capacity towards heat. Moreover, PVC is a polymer which is made from polymerization of vinyl chloride and is used in products such as pipes, bottles etc.
Complete step by step answer:
A polymer is a large molecule or a macromolecule which is further a combination of many subunits. They can be naturally found in plants and animals or are man-made. Basically, there are three types of polymers:
1.Natural
2.Synthetic
3.Semi-synthetic
Basically, when two different types of monomers are joined together in the same polymer chain, then the polymer formed is known as a co-polymer. Moreover, the process in which a polymer is formed from multiple species of monomers, is known as copolymerization and the copolymers obtained by copolymerization of two monomer species are called as biopolymers.
Now, co- polymers are classified as:
1.Linear copolymers (single chain)
2.Branched copolymers (polymeric chain)
3.Cross-linked polymer (composed of bifunctional and trifunctional monomers)
The structures are as shown:

Now, among the given options Buna-S is a copolymer. It is made up of two monomers i.e. 1,3- butadiene and Styrene. It is also known as Styrene-Butadiene Rubber. It is used for making automobile tyres and rubber soles. The polymerization is as shown:

Hence, option A is correct.
Note: Teflon is made up of a chemical compound named polytetrafluoroethylene which is a synthetic fluoropolymer. This compound is used as the coating for non-stick pans as they are hydrophobic and have a high resistance capacity towards heat. Moreover, PVC is a polymer which is made from polymerization of vinyl chloride and is used in products such as pipes, bottles etc.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding How a Current Loop Acts as a Magnetic Dipole

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Atomic Structure for Beginners

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis




