
Which of the following is a red liquid?
A. $S{F_4}$
B. $S{F_6}$
C. $SC{l_2}$
D. ${S_2}C{l_2}$
Answer
233.1k+ views
Hint: A chemical gets its color by electrons that absorb energy and become excited. The colour basically comes from the excitation of electrons due to the absorption of energy performed by the chemical. Red liquid generally refers to a solution that has a red color and is liquid at room temperature.
Complete step by step answer:
Basically a red liquid is the one which has a reddish- brown appearance and is liquid at room temperature.
Among the given options $SC{l_2}$ (Sulphur dichloride) is considered as a red liquid because it is red in colour and is liquid at room temperature. This cherry-red liquid is the simplest sulfur chloride and is most commonly used.
The structure of the compound is as shown:
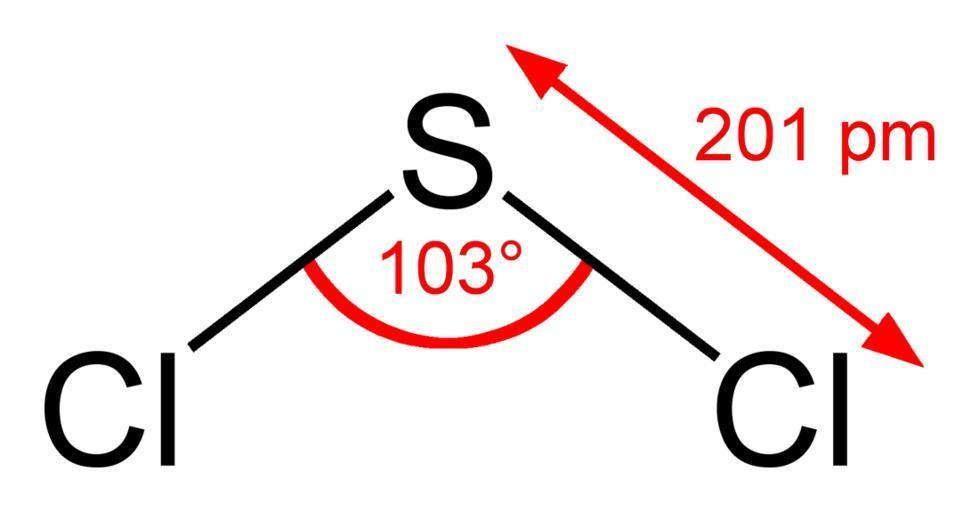
As shown in the figure, it seems to have a bent shape with asymmetric charge distribution. It further contains one Sulphur and two chlorine molecules. It has a bond angle of ${103^ \circ }$ and bond length of $201pm$
\
Hence, option C is correct.
Note: $S{F_4}$ I.e. Sulphur tetrafluoride is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture, $S{F_6}$ i.e. sulfur hexafluoride is also colorless,odorless, non-flammable and non-toxic gas and ${S_2}C{l_2}$ i.e. disulphur dichloride is a light-amber to yellow oily liquid.
Complete step by step answer:
Basically a red liquid is the one which has a reddish- brown appearance and is liquid at room temperature.
Among the given options $SC{l_2}$ (Sulphur dichloride) is considered as a red liquid because it is red in colour and is liquid at room temperature. This cherry-red liquid is the simplest sulfur chloride and is most commonly used.
The structure of the compound is as shown:

As shown in the figure, it seems to have a bent shape with asymmetric charge distribution. It further contains one Sulphur and two chlorine molecules. It has a bond angle of ${103^ \circ }$ and bond length of $201pm$
\
Hence, option C is correct.
Note: $S{F_4}$ I.e. Sulphur tetrafluoride is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture, $S{F_6}$ i.e. sulfur hexafluoride is also colorless,odorless, non-flammable and non-toxic gas and ${S_2}C{l_2}$ i.e. disulphur dichloride is a light-amber to yellow oily liquid.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




