
Which of the following has the highest dipole moment?
(A) \[C{H_3}Cl\]
(B) \[C{H_3}F\]
(C) \[C{H_3}Br\]
(D) \[C{H_3}I\]
Answer
233.1k+ views
Hint: Polar chemicals have a dipole moment other than zero, while non-polar compounds have a dipole moment of zero. The net dipole moment in a molecule will be 0 if the identical atoms are linearly present in front of one another, linked to the centre atom, and have the same magnitude but opposite direction of the dipole.
Complete Step by Step Solution:
We must first comprehend the idea of a dipole moment before we can compare the dipole moments of the supplied molecules.
When there is a charge separation, a phenomenon known as the dipole moment develops. Dipole moments are brought into play by the differences in electronegativity and can be seen between two different ions connected by ionic connections or it can be seen between atoms connected by covalent bonds. The dipole moment also rises as the difference in electronegativity widens. The dipole moment's magnitude is also influenced by the distance between the positive and negative charge separations. The magnitude of the dipole moment serves as a gauge for the polarity that a molecule possesses.
Now, in order to comprehend the dipole moments, we will examine each answer choice and sketch corresponding Lewis dot structures. The Lewis dot structure of chloromethane is displayed below if we take the first choice, which says either chloromethane or methyl chloride:
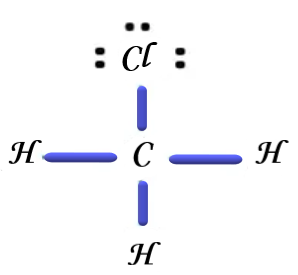
(Self made)
Now if we consider the second option, \[C{H_3}F\] is a non-toxic and liquefiable gas also known as Fluor-methane or methyl fluoride. It is a colourless and flammable gas. The Lewis dot structure of Fluor-methane is drawn below,
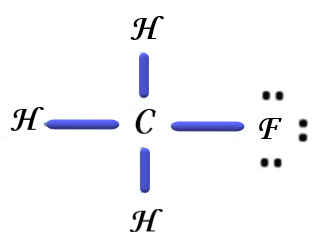
Now if we consider the third option, the Lewis dot structure of Bromomethane is drawn below,
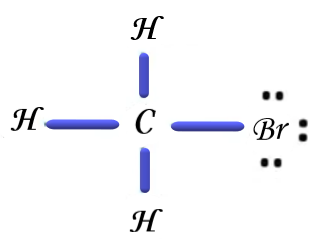
Similarly, if we consider the last option, the Lewis dot structure of Iodomethane, that is also called methyl iodide,
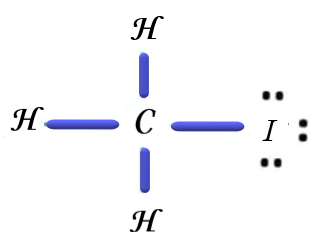
So, after comparing the four structures, we can see that the dipole moment of methyl chloride is maximum among the four.
The correct order of dipole moments is \[C{H_3}Cl > C{H_3}F > C{H_3}Br > C{H_3}I\].
Therefore, the correct option is: (A) \[C{H_3}Cl\]
Note: Among fluor-methane, bromomethane, and iodomethane, methyl chloride has the highest dipole moment because its bond length is larger while having lower charges, and its product of charges and bond length is more than that of methyl fluoride.
Complete Step by Step Solution:
We must first comprehend the idea of a dipole moment before we can compare the dipole moments of the supplied molecules.
When there is a charge separation, a phenomenon known as the dipole moment develops. Dipole moments are brought into play by the differences in electronegativity and can be seen between two different ions connected by ionic connections or it can be seen between atoms connected by covalent bonds. The dipole moment also rises as the difference in electronegativity widens. The dipole moment's magnitude is also influenced by the distance between the positive and negative charge separations. The magnitude of the dipole moment serves as a gauge for the polarity that a molecule possesses.
Now, in order to comprehend the dipole moments, we will examine each answer choice and sketch corresponding Lewis dot structures. The Lewis dot structure of chloromethane is displayed below if we take the first choice, which says either chloromethane or methyl chloride:

(Self made)
Now if we consider the second option, \[C{H_3}F\] is a non-toxic and liquefiable gas also known as Fluor-methane or methyl fluoride. It is a colourless and flammable gas. The Lewis dot structure of Fluor-methane is drawn below,

Now if we consider the third option, the Lewis dot structure of Bromomethane is drawn below,

Similarly, if we consider the last option, the Lewis dot structure of Iodomethane, that is also called methyl iodide,

So, after comparing the four structures, we can see that the dipole moment of methyl chloride is maximum among the four.
The correct order of dipole moments is \[C{H_3}Cl > C{H_3}F > C{H_3}Br > C{H_3}I\].
Therefore, the correct option is: (A) \[C{H_3}Cl\]
Note: Among fluor-methane, bromomethane, and iodomethane, methyl chloride has the highest dipole moment because its bond length is larger while having lower charges, and its product of charges and bond length is more than that of methyl fluoride.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




