
Which of the following has the highest boiling point?
A.${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}$
B.${\rm{C}}{{\rm{H}}_3}{\rm{COOH}}$
C.${\rm{C}}{{\rm{H}}_3}{\rm{COC}}{{\rm{H}}_{\rm{3}}}$
D.${{\rm{C}}_{\rm{2}}}{{\rm{H}}_6}$
Answer
240.6k+ views
Hint:To decide which molecule has the highest boiling point, first we have to understand the factors that affect the boiling point. The formation of hydrogen bonding between molecules affects the boiling point.
Complete step-by-step answer:
Hydrocarbons have lower boiling points than alcohols because of absence of intermolecular hydrogen bonding. Ethers also have lower boiling point than alcohol because of absence of hydrogen bonding. Carboxylic acid has more boiling point than alcohol because they can form dimer.
Let’s find the correct option.
The given compounds are ethanol $\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}} \right)$, ethanoic acid $\left( {{\rm{C}}{{\rm{H}}_3}{\rm{COOH}}} \right)$, methoxymethane$\left( {{\rm{C}}{{\rm{H}}_3}{\rm{COC}}{{\rm{H}}_{\rm{3}}}} \right)$ and ethane $\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_6}} \right)$. As no intermolecular H-bonding present in case of ethane and methoxymethane, they have lower boiling point. Now the remaining two compounds are ethanol and ethanoic acid. Both can form intermolecular H-bond. But, ethanoic acid can form dimmer. The dimer forms because of the presence of H-bonding between carbonyl oxygen and acidic hydrogen. The dimer formation can be shown as below.
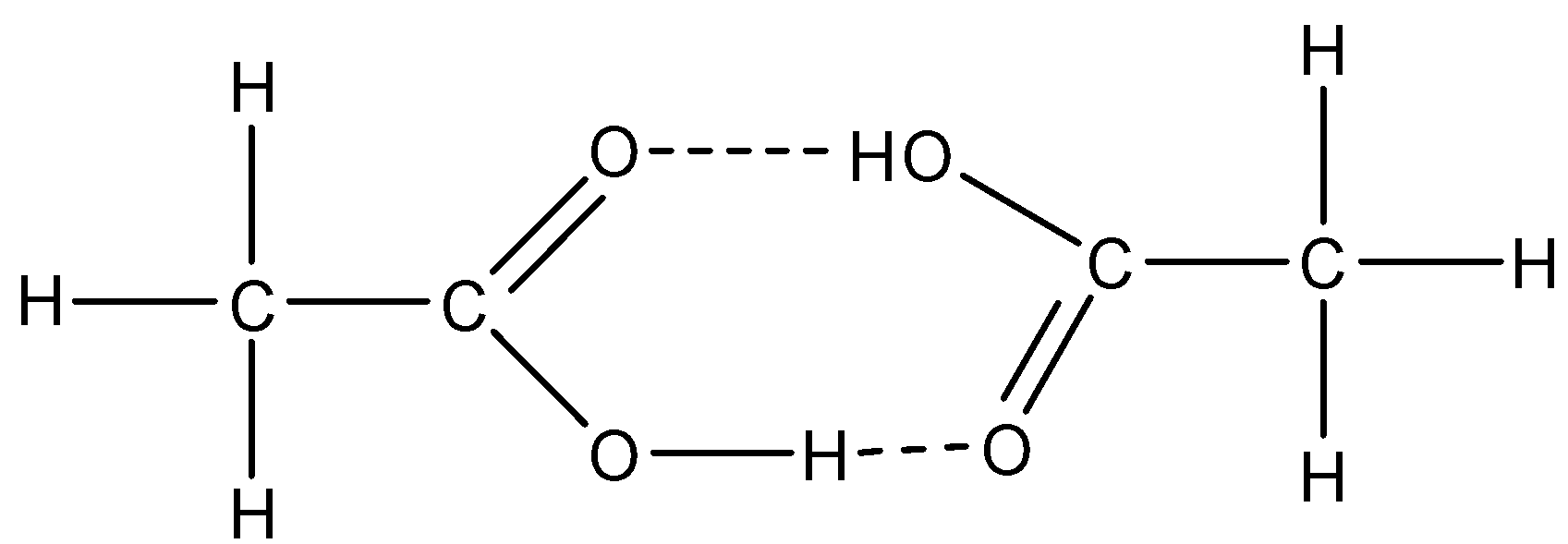
Due to formation of dimmers, the van der Waals force increases, due to which the boiling point increases. So, the molecule whose boiling point is highest is ethanoic acid.
Hence, the correct option is B.
Addition Information:
A chemical bond where a hydrogen atom forms covalent bond with electronegative atoms like fluorine, nitrogen and oxygen is called hydrogen bond. There are two types of hydrogen bonding, intermolecular hydrogen bonding and intramolecular hydrogen bonding.
If the H-bonding occurs within the molecule, then this type of bonding is intramolecular H-bonding and if the H-bonding occurs between two same or different molecules, then this type of bonding is intermolecular H-bonding.
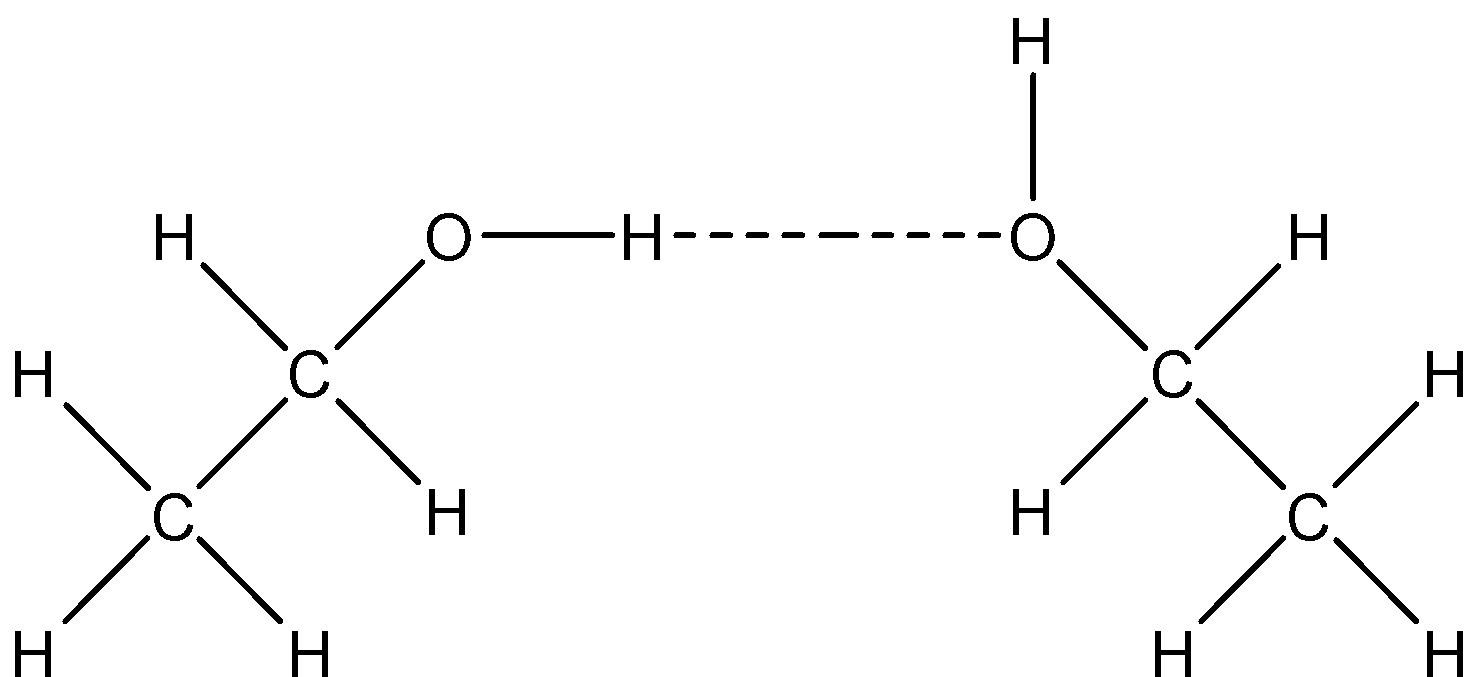
Note: The alcohols do not form dimers like carboxylic acid. The intermolecular H-bonding of ethanol can be shown as below:
Complete step-by-step answer:
Hydrocarbons have lower boiling points than alcohols because of absence of intermolecular hydrogen bonding. Ethers also have lower boiling point than alcohol because of absence of hydrogen bonding. Carboxylic acid has more boiling point than alcohol because they can form dimer.
Let’s find the correct option.
The given compounds are ethanol $\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}} \right)$, ethanoic acid $\left( {{\rm{C}}{{\rm{H}}_3}{\rm{COOH}}} \right)$, methoxymethane$\left( {{\rm{C}}{{\rm{H}}_3}{\rm{COC}}{{\rm{H}}_{\rm{3}}}} \right)$ and ethane $\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_6}} \right)$. As no intermolecular H-bonding present in case of ethane and methoxymethane, they have lower boiling point. Now the remaining two compounds are ethanol and ethanoic acid. Both can form intermolecular H-bond. But, ethanoic acid can form dimmer. The dimer forms because of the presence of H-bonding between carbonyl oxygen and acidic hydrogen. The dimer formation can be shown as below.

Due to formation of dimmers, the van der Waals force increases, due to which the boiling point increases. So, the molecule whose boiling point is highest is ethanoic acid.
Hence, the correct option is B.
Addition Information:
A chemical bond where a hydrogen atom forms covalent bond with electronegative atoms like fluorine, nitrogen and oxygen is called hydrogen bond. There are two types of hydrogen bonding, intermolecular hydrogen bonding and intramolecular hydrogen bonding.
If the H-bonding occurs within the molecule, then this type of bonding is intramolecular H-bonding and if the H-bonding occurs between two same or different molecules, then this type of bonding is intermolecular H-bonding.
Note: The alcohols do not form dimers like carboxylic acid. The intermolecular H-bonding of ethanol can be shown as below:

Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

Inductive Effect and Its Role in Acidic Strength




