
Which of the following has electron deficient compounds?
(A) ${B_2}{H_6}$, $AlC{l_3}$
(B) ${C_2}{H_6}$, $A{l_2}C{l_6}$
(C) $S{F_2}$, $C{l_2}O$
(D) $NaB{H_4}$, $ICl$
Answer
233.4k+ views
Hint: An electron deficient compound is the one in which there is an insufficient number of electrons to complete the octet of the central atom. These compounds contain insufficient numbers of electrons to form normal electron-pair bonds between each pair of the bonded atoms.
Complete step by step solution:
${B_2}{H_6}$ (Diborane) and $AlC{l_3}$ are electron deficient compounds. This is because:
In case of diborane, (as shown in the structure), each boron bonds with 4 hydrogen atoms.
But the valency of boron is 3 and each boron gives 3 valence electrons.
But in case of the 4th bond, there is no electron from boron’s side and hence making it electron deficient.
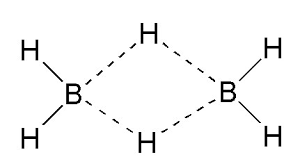
Now,
In case of $AlC{l_3}$ (as shown), Al atom bonds with $3$ Cl atoms. But it violates the octet rule. There are no bonds or lone pairs, which makes the surrounding electron count 8. It has only 3 bonds which makes only 6 electrons and hence making it an electron deficient compound.
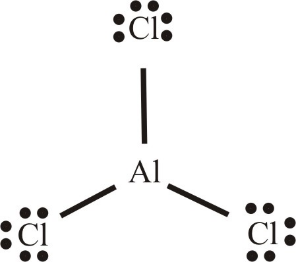
Therefore, ${B_2}{H_6}$ and $AlCl{}_3$ both are electron deficient compounds.
Hence, option A is correct.
Note: Diborane is used in rocket propellants. It is also used as a rubber vulcanizer and a catalyst for olefin polymerization whereas aluminium chloride is used in the pharmaceutical industry. It is also used as an antiperspirant in products such as Drysol.
Complete step by step solution:
${B_2}{H_6}$ (Diborane) and $AlC{l_3}$ are electron deficient compounds. This is because:
In case of diborane, (as shown in the structure), each boron bonds with 4 hydrogen atoms.
But the valency of boron is 3 and each boron gives 3 valence electrons.
But in case of the 4th bond, there is no electron from boron’s side and hence making it electron deficient.

Now,
In case of $AlC{l_3}$ (as shown), Al atom bonds with $3$ Cl atoms. But it violates the octet rule. There are no bonds or lone pairs, which makes the surrounding electron count 8. It has only 3 bonds which makes only 6 electrons and hence making it an electron deficient compound.

Therefore, ${B_2}{H_6}$ and $AlCl{}_3$ both are electron deficient compounds.
Hence, option A is correct.
Note: Diborane is used in rocket propellants. It is also used as a rubber vulcanizer and a catalyst for olefin polymerization whereas aluminium chloride is used in the pharmaceutical industry. It is also used as an antiperspirant in products such as Drysol.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




