
Which of the following does not form intramolecular hydrogen bonding?
A. \[{\rm{CC}}{{\rm{l}}_{\rm{3}}}{\rm{CH(OH}}{{\rm{)}}_{\rm{2}}}\]
B.
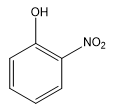
C.
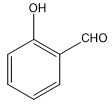
D.
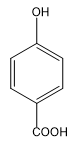
Answer
233.1k+ views
Hint: Hydrogen bonding is a special type of dipole-dipole interaction and exists in molecules containing a hydrogen atom linked by a covalent bond to a highly electronegative atom having a small size.
Complete Step by Step Solution:
The electrostatic force of attraction between the hydrogen atom of one molecule and a highly electronegative element such as \[{\rm{N,}}\,{\rm{O}}\]and \[{\rm{F}}\] present within the same molecule or another molecule of the same or different compounds is known as hydrogen bond.
A hydrogen bond is represented by a dotted line in contrast to a thick line representing a covalent bond between two atoms. There are two different types of hydrogen bonds. They are:
(i) Intermolecular hydrogen bonding: This type of hydrogen bond is formed between the two molecules of the same or different compounds. Some examples of the compounds exhibiting intermolecular hydrogen bonds are hydrogen fluoride \[{\rm{(HF)}}\], water \[{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{O)}}\], and ammonia \[{\rm{(N}}{{\rm{H}}_3})\].
(ii) Intramolecular hydrogen bonding: This type of hydrogen bond is formed between hydrogen atom and \[{\rm{N,}}\,{\rm{O}}\]and \[{\rm{F}}\].atom of the same molecule. This type of bonding is commonly known as chelation and is more frequently found in organic compounds. Intramolecular hydrogen bonding is possible when six or five-membered rings can be formed. Some examples are ortho-nitro phenol, salicylic acid and salicylaldehyde.
The compound in option (A) is 2,2,2-trichloroethane-1,1-diol, which is also called a chloral hydrate. The chlorine atom is highly electronegative and is known to not show any hydrogen bonding either inter or intra because of its electron density that comes from its size. Chloral hydrate is a geminal diol which is usually unstable. In order to make it stable, chlorine is believed to form intramolecular hydrogen bonding.
The compound in option (B) is ortho-nitrophenol. Chelation (intramolecular hydrogen bonding) is only possible in ortho isomers since the two groups are close to each other. Hence, intramolecular hydrogen bonding takes place in ortho-nitrophenol.
The compound in option (C) is salicylaldehyde. Chelation (intramolecular hydrogen bonding) is only possible in ortho isomers since the two groups are close to each other. Hence, intramolecular hydrogen bonding takes place in salicylaldehyde.
The compound din option (D) is para- hydroxybenzoic acid. Chelation (intramolecular hydrogen bonding) is not possible in meta and para isomers since the two groups are located far away from each other. Therefore, in such cases intermolecular hydrogen bonding takes place.
Therefore, option D is correct.
Note: Intramolecular hydrogen bonding (chelation) decreases the boiling point of the compound and also its solubility in water by restricting the possibility of intermolecular hydrogen bonding.
Complete Step by Step Solution:
The electrostatic force of attraction between the hydrogen atom of one molecule and a highly electronegative element such as \[{\rm{N,}}\,{\rm{O}}\]and \[{\rm{F}}\] present within the same molecule or another molecule of the same or different compounds is known as hydrogen bond.
A hydrogen bond is represented by a dotted line in contrast to a thick line representing a covalent bond between two atoms. There are two different types of hydrogen bonds. They are:
(i) Intermolecular hydrogen bonding: This type of hydrogen bond is formed between the two molecules of the same or different compounds. Some examples of the compounds exhibiting intermolecular hydrogen bonds are hydrogen fluoride \[{\rm{(HF)}}\], water \[{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{O)}}\], and ammonia \[{\rm{(N}}{{\rm{H}}_3})\].
(ii) Intramolecular hydrogen bonding: This type of hydrogen bond is formed between hydrogen atom and \[{\rm{N,}}\,{\rm{O}}\]and \[{\rm{F}}\].atom of the same molecule. This type of bonding is commonly known as chelation and is more frequently found in organic compounds. Intramolecular hydrogen bonding is possible when six or five-membered rings can be formed. Some examples are ortho-nitro phenol, salicylic acid and salicylaldehyde.
The compound in option (A) is 2,2,2-trichloroethane-1,1-diol, which is also called a chloral hydrate. The chlorine atom is highly electronegative and is known to not show any hydrogen bonding either inter or intra because of its electron density that comes from its size. Chloral hydrate is a geminal diol which is usually unstable. In order to make it stable, chlorine is believed to form intramolecular hydrogen bonding.
The compound in option (B) is ortho-nitrophenol. Chelation (intramolecular hydrogen bonding) is only possible in ortho isomers since the two groups are close to each other. Hence, intramolecular hydrogen bonding takes place in ortho-nitrophenol.
The compound in option (C) is salicylaldehyde. Chelation (intramolecular hydrogen bonding) is only possible in ortho isomers since the two groups are close to each other. Hence, intramolecular hydrogen bonding takes place in salicylaldehyde.
The compound din option (D) is para- hydroxybenzoic acid. Chelation (intramolecular hydrogen bonding) is not possible in meta and para isomers since the two groups are located far away from each other. Therefore, in such cases intermolecular hydrogen bonding takes place.
Therefore, option D is correct.
Note: Intramolecular hydrogen bonding (chelation) decreases the boiling point of the compound and also its solubility in water by restricting the possibility of intermolecular hydrogen bonding.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




