
Which of the following conditions is used to find atomic \[C{{l}_{2}}\] from molecular \[C{{l}_{2}}\]?
(A) High temperature, high pressure
(B) Low temperature, high pressure
(C) High temperature, low pressure
(D) Low temperature, low pressure
Answer
233.1k+ views
Hint: Molecule \[C{{l}_{2}}\] means both chlorine atoms bonded to each other through a covalent bond (both are non-metal) whereas atomic \[C{{l}_{2}}\] means both chlorine atoms are not bonded. For conversion, molecular chlorine to atomic chlorine ∆H should be greater than zero.
Complete Step by Step Answer:
In molecular chlorine, two chlorine atoms are bonded with a covalent bond and a covalent bond are of two types one is polar and the other is non-polar. As there is no electronegativity difference between two chlorine atoms, the bond is nonpolar.
Now to convert molecular chlorine to atomic chlorine this bond must be broken. Thus, we need to give bond dissociation energy due to this, ∆H will be positive or greater than zero. This is the condition of endothermic reaction in which we need to provide a high temperature to break the bond (heat is evolved) such as
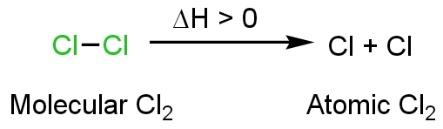
High-temperature conditions favour the forward reaction. Other conditions of endothermic reaction of high pressure but high-pressure favour backward reaction. Thus, high pressure is not a good condition for the conversion of molecular chlorine to atomic chlorine. So it will be low pressure which favours the forward reaction (formation of atomic chlorine) such as

Thus, the correct option is C.
Note: At high-pressure reaction proceed to that direction in which less number of moles of gas is present (favour backward reaction) at equilibrium. But as per the given question, we need the condition that favours forward reaction and result in formation of atomic chlorine.
Complete Step by Step Answer:
In molecular chlorine, two chlorine atoms are bonded with a covalent bond and a covalent bond are of two types one is polar and the other is non-polar. As there is no electronegativity difference between two chlorine atoms, the bond is nonpolar.
Now to convert molecular chlorine to atomic chlorine this bond must be broken. Thus, we need to give bond dissociation energy due to this, ∆H will be positive or greater than zero. This is the condition of endothermic reaction in which we need to provide a high temperature to break the bond (heat is evolved) such as
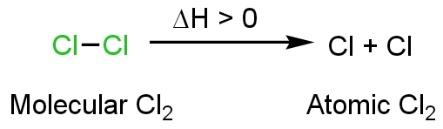
High-temperature conditions favour the forward reaction. Other conditions of endothermic reaction of high pressure but high-pressure favour backward reaction. Thus, high pressure is not a good condition for the conversion of molecular chlorine to atomic chlorine. So it will be low pressure which favours the forward reaction (formation of atomic chlorine) such as

Thus, the correct option is C.
Note: At high-pressure reaction proceed to that direction in which less number of moles of gas is present (favour backward reaction) at equilibrium. But as per the given question, we need the condition that favours forward reaction and result in formation of atomic chlorine.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




