
Which of the following cannot undergo a $E2$ reaction?
(A)
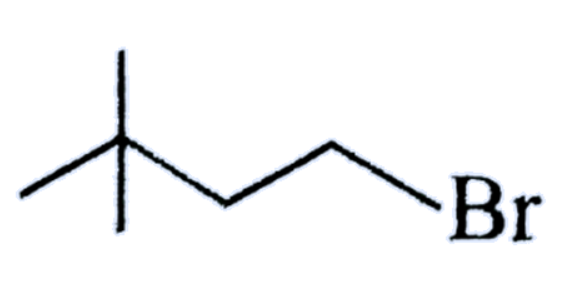
(B)
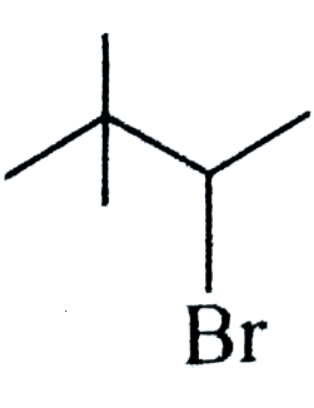
(C)
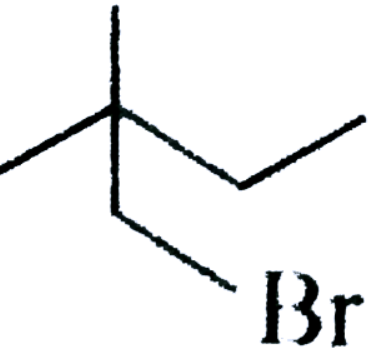
(D) None of these



Answer
233.1k+ views
Hint: In this question we have to find that which of the above given option didn’t perform elimination reaction. For finding the option which didn’t perform elimination reaction we have to know all the terms which help us to know how a elimination reaction perform that we are going to study in the solution.
Complete Step by Step Solution:
As we know that, in the options there are three types of alkyl halides are given and we also know that if any compound having halogen and hydrogen atom on their $\beta - carbon$ atom then that compound can form elimination reaction.
So, we have to see that which of the above compound does not have a $\beta - carbon$ atom by which we can say that reaction will not perform elimination reaction,
So, by doing further reaction we get that,
In the (C) option there is an absence of hydrogen atom in $\beta - carbon$ by which we can say, that the compound cannot perform an elimination reaction.
Therefore, we can say that option (C) cannot undergo a $E2$ reaction.
Hence, the correct option is (C).
Note: This question is taken from Haloalkanes and Haloarenes. So, let’s we know that what are haloalkanes and haloarenes. Haloalkanes are those which contains alkanes with having halogens as in their substituents and in Haloarenes are those when any hydrogen atom is bonded to the benzene ring and got replaced by the halogen atoms and the compound which form from the whole reaction the compound is called as by the name of Haloarenes.
Complete Step by Step Solution:
As we know that, in the options there are three types of alkyl halides are given and we also know that if any compound having halogen and hydrogen atom on their $\beta - carbon$ atom then that compound can form elimination reaction.
So, we have to see that which of the above compound does not have a $\beta - carbon$ atom by which we can say that reaction will not perform elimination reaction,
So, by doing further reaction we get that,
In the (C) option there is an absence of hydrogen atom in $\beta - carbon$ by which we can say, that the compound cannot perform an elimination reaction.
Therefore, we can say that option (C) cannot undergo a $E2$ reaction.
Hence, the correct option is (C).
Note: This question is taken from Haloalkanes and Haloarenes. So, let’s we know that what are haloalkanes and haloarenes. Haloalkanes are those which contains alkanes with having halogens as in their substituents and in Haloarenes are those when any hydrogen atom is bonded to the benzene ring and got replaced by the halogen atoms and the compound which form from the whole reaction the compound is called as by the name of Haloarenes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




