
What are D and L isomers?
Answer
232.8k+ views
Hint: Isomers are compounds that have the same molecular formulas but different structural formulae, and this phenomenon is known as isomerism. Isomers differ not only in structural formula but also in physical and chemical characteristics.
Complete Step by Step Solution:
The position of the second last hydroxyl group in carbohydrates is represented by the letters D and L.
The symbol D denotes a carbohydrate with the hydroxyl group at the last chiral carbon on the right side, whereas the letter L denotes a carbohydrate with the hydroxyl group at the last chiral carbon on the left side.
Let's use glucose as an example of a carbohydrate. D-(+)-Glucose is the right term for glucose. L-(+)-Glucose is the enantiomer of glucose, which is optically active.
The letters D and L preceding the name of glucose denote the hydroxyl group's arrangement at the penultimate carbon atom, i.e., the carbon chain's last but one carbon atom.
The algebraic signs '+' and '-' within brackets after the letters D and L, on the other hand, represent optical rotation signs. Or, to put it another way, dextrorotatory and laevorotatory, respectively.
The letters D and L have nothing to do with the optical rotation indication. This indicates that a carbohydrate with the letter 'D' can be dextrorotatory or laevorotatory, and carbohydrate with the letter 'L' can also be dextrorotatory or laevorotatory. D-glucose, for example, is dextrorotatory, whereas D- fructose is laevorotatory.
Glyceraldehyde can be used to explain the significance of D and L configurations. The standard for assigning D and L configurations to monosaccharides is glyceraldehyde. It is the most basic carbohydrate because it just has one chiral carbon.
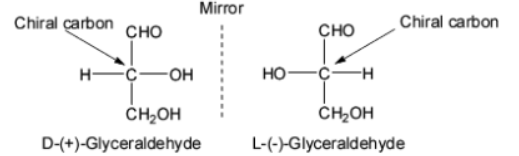
Image: D and L isomers of Glyceraldehyde
As a result, all monosaccharides associated with the '+' isomer of glyceraldehyde are assigned the D configuration, while those correlated to the '-' isomer are allocated the L configuration.
Note: The structures of monosaccharides are written so that the most oxidised carbon, i.e. the aldehyde group, is at the top when assigning these configurations.
Thus, (+) glucose is allocated the D configuration, while (-) glucose is assigned the L configuration, according to this debate.
Complete Step by Step Solution:
The position of the second last hydroxyl group in carbohydrates is represented by the letters D and L.
The symbol D denotes a carbohydrate with the hydroxyl group at the last chiral carbon on the right side, whereas the letter L denotes a carbohydrate with the hydroxyl group at the last chiral carbon on the left side.
Let's use glucose as an example of a carbohydrate. D-(+)-Glucose is the right term for glucose. L-(+)-Glucose is the enantiomer of glucose, which is optically active.
The letters D and L preceding the name of glucose denote the hydroxyl group's arrangement at the penultimate carbon atom, i.e., the carbon chain's last but one carbon atom.
The algebraic signs '+' and '-' within brackets after the letters D and L, on the other hand, represent optical rotation signs. Or, to put it another way, dextrorotatory and laevorotatory, respectively.
The letters D and L have nothing to do with the optical rotation indication. This indicates that a carbohydrate with the letter 'D' can be dextrorotatory or laevorotatory, and carbohydrate with the letter 'L' can also be dextrorotatory or laevorotatory. D-glucose, for example, is dextrorotatory, whereas D- fructose is laevorotatory.
Glyceraldehyde can be used to explain the significance of D and L configurations. The standard for assigning D and L configurations to monosaccharides is glyceraldehyde. It is the most basic carbohydrate because it just has one chiral carbon.

Image: D and L isomers of Glyceraldehyde
As a result, all monosaccharides associated with the '+' isomer of glyceraldehyde are assigned the D configuration, while those correlated to the '-' isomer are allocated the L configuration.
Note: The structures of monosaccharides are written so that the most oxidised carbon, i.e. the aldehyde group, is at the top when assigning these configurations.
Thus, (+) glucose is allocated the D configuration, while (-) glucose is assigned the L configuration, according to this debate.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




