
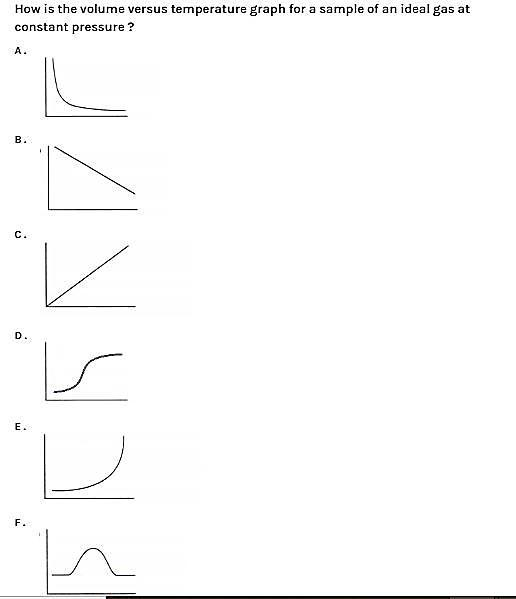
How is the volume versus temperature graph for a sample of an ideal gas at constant pressure?
Answer
239.4k+ views
Hint: To answer this question, we should know about the relationship between volume and temperature. This relationship was given by Charles’ law. By using this law we can easily find out the answer.
Complete step by step answer:
> To answer this question, we should know about Charles’ law. We should know that Charles’ law is one of the gas laws which explain the relationship between volume and temperature of a gas. Charles’s law states that when pressure is held constant, the volume of a fixed amount of dry gas is directly proportional to its absolute temperature. It should be noted that, when we take two measurements in direct proportion then any change made in one of them affects the other through direct variation. Charles’ Law is expressed by the equation:
\[\begin{align}
& V\alpha T \\
& \dfrac{V1}{T1}=\dfrac{V2}{T2} \\
\end{align}\]
> From the above expression we should know that V1 and V2 are the Initial Volumes and Final Volume respectively. T1 refers to the Initial Temperature and T2 refers to the Final Temperature.
> We can understand this by taking one example, we should know that on heating up a fixed mass of gas, that is, increasing the temperature, the volume also increases. Similarly, on cooling, the volume of the gas decreases.
So, from this we can now say that option C is correct. In this graph we can say that it is a straight line because volume and temperature are directly proportional.
Note: It is important to discuss that the unit Kelvin is preferred for solving problems related to Charles Law, and not Celsius. Kelvin (T) is also known as the Absolute temperature scale. We should know about Charles' law application in real life. It has a wide range of applications. Helium balloons shrink in a cold environment. Second example is this : while jogging in winters, we face difficulty because our lung capacity decreases.
Complete step by step answer:
> To answer this question, we should know about Charles’ law. We should know that Charles’ law is one of the gas laws which explain the relationship between volume and temperature of a gas. Charles’s law states that when pressure is held constant, the volume of a fixed amount of dry gas is directly proportional to its absolute temperature. It should be noted that, when we take two measurements in direct proportion then any change made in one of them affects the other through direct variation. Charles’ Law is expressed by the equation:
\[\begin{align}
& V\alpha T \\
& \dfrac{V1}{T1}=\dfrac{V2}{T2} \\
\end{align}\]
> From the above expression we should know that V1 and V2 are the Initial Volumes and Final Volume respectively. T1 refers to the Initial Temperature and T2 refers to the Final Temperature.
> We can understand this by taking one example, we should know that on heating up a fixed mass of gas, that is, increasing the temperature, the volume also increases. Similarly, on cooling, the volume of the gas decreases.
So, from this we can now say that option C is correct. In this graph we can say that it is a straight line because volume and temperature are directly proportional.
Note: It is important to discuss that the unit Kelvin is preferred for solving problems related to Charles Law, and not Celsius. Kelvin (T) is also known as the Absolute temperature scale. We should know about Charles' law application in real life. It has a wide range of applications. Helium balloons shrink in a cold environment. Second example is this : while jogging in winters, we face difficulty because our lung capacity decreases.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
Difference Between Crystalline and Amorphous Solid

Understanding Electromagnetic Waves and Their Importance

Common Ion Effect: Concept, Applications, and Problem-Solving

Correct order of basic strength of given amines is class 11 chemistry JEE_Main

Understanding the Electric Field of a Charged Spherical Shell

Understanding How a Current Loop Acts as a Magnetic Dipole

Other Pages
Understanding the Electric Field Due to Infinite Linear Charge and Cylinders

JEE Main Colleges 2026: Complete List of Participating Institutes

Understanding Collisions: Types and Examples for Students

How Does Fusion Reaction Happen Inside the Sun?

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding EMF and Internal Resistance of a Cell




