
Van der Waals' forces are applied to:
A) Inert gases only
B) Rate gases only
C) Mixture of gases
D) Elementary gases only
Answer
232.8k+ views
Hint: Van der Waals's force of attraction is the intermolecular forces that exist between the molecules. The molecules develop the charges through the induced dipole moment. The charge developed can be either permanent or temporary.
Complete step by step answer:
Van der Waals forces are used to define the intermolecular forces between the molecules. There are two types of Van der Waals forces:
1) Strong such as the dipole-dipole moment
2) Weak such as London-London dispersion forces
-In a molecule, the accumulation of electrons in a part of an atom as an electron cloud is called the electron charge density. The movement of all electrons in a direction leads to the formation of the dipole, where the one end of the atom is a highly negative charge and the other is relatively less negative. Even if the molecule is nonpolar the moment of electrons in presence of other atoms makes the non-polar molecule form poles for a moment.
-For a polar molecule, since the electrons are concentrated at the one end the molecules partially gain the negative charge in the end. This negative end has a dipole such that it can attract the surrounding molecule.
The ability of molecules to form dipoles and displace its electrons is known as the polarizability.
The different polar or nonpolar molecules experience the van der Waals force of attraction.
1) Dipole-dipole interaction: This interaction occurs between the molecules which have the permanent dipole moment. These are also referred to as polar molecules. For example, \[\text{HCl}\], \[\text{HF}\] etc. Here, the one-electron density is already at the one end of the molecules. The \[\text{HCl}\] electron density moves from $\text{H}$ to $\text{Cl}$ creating a dipole on molecules that attracts the other molecules from the surrounding towards it. It is shown in the following figure
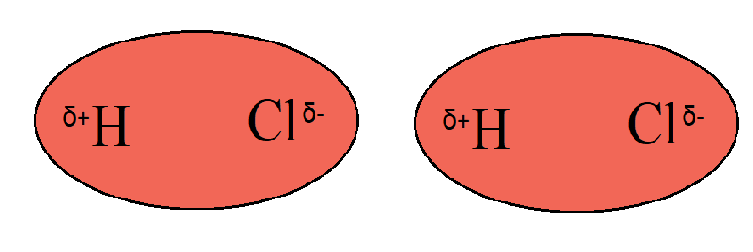
2) Dipole Induced dipoles forces exist between the neutral polar molecules. This is the interaction of a molecule containing permanent dipole moment and with the molecule having no permanent dipole. For example, the non-polar molecule undergoes the separation of charges as another polar molecule approaches towards it.
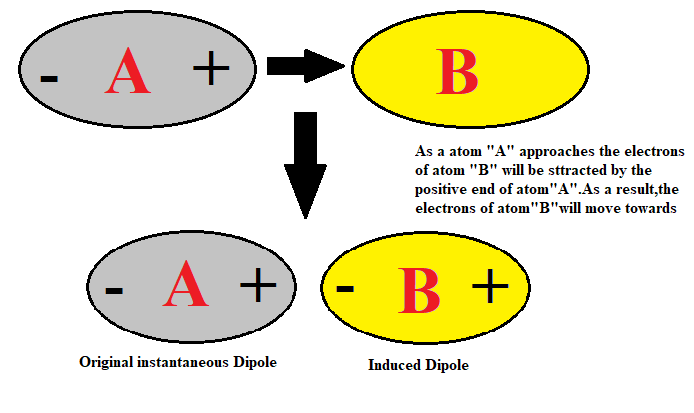
3) London forces of attraction: The molecules which have the induced dipole can induce the dipole in neighbouring molecules. This forms the dipoles due to the induced dipole interaction. The London force of attraction exists in the gaseous molecules. London-London dispersion forces exist in helium. These are forces between nonpolar molecules. This forces are found in ${{\text{F}}_{\text{2}}}$, ${{\text{I}}_{\text{2}}}$
Van der Waals forces of attraction exist between the inert gases or Noble gases, elementary gases and between the rate gases. Therefore, we can say that the van der Waals force is applicable to a mixture of gases.
Hence, the correct option is (C).
Additional information:
The strength of Van der Waals forces
This forces depend on the size and shape of molecule
1) Size :In periodic tables, the boiling point of Noble gases increases as we move down in groups. This is because the radius of the atoms increases. Therefore larger the atomic radii higher is distance and greater possibility of polarization. Therefore bigger molecules have higher boiling points.
2) Shape of molecules is an important strength of force. Long and thin molecules are capable of developing dipoles than that of the fat and short molecules.
Note: Van der Waals forces are distance dependent forces. The interaction is maximum when the distance between the molecules is less. Gas particles interact with each other thus one of the above forces is always present in inert, rate or elementary gas.
Complete step by step answer:
Van der Waals forces are used to define the intermolecular forces between the molecules. There are two types of Van der Waals forces:
1) Strong such as the dipole-dipole moment
2) Weak such as London-London dispersion forces
-In a molecule, the accumulation of electrons in a part of an atom as an electron cloud is called the electron charge density. The movement of all electrons in a direction leads to the formation of the dipole, where the one end of the atom is a highly negative charge and the other is relatively less negative. Even if the molecule is nonpolar the moment of electrons in presence of other atoms makes the non-polar molecule form poles for a moment.
-For a polar molecule, since the electrons are concentrated at the one end the molecules partially gain the negative charge in the end. This negative end has a dipole such that it can attract the surrounding molecule.
The ability of molecules to form dipoles and displace its electrons is known as the polarizability.
The different polar or nonpolar molecules experience the van der Waals force of attraction.
1) Dipole-dipole interaction: This interaction occurs between the molecules which have the permanent dipole moment. These are also referred to as polar molecules. For example, \[\text{HCl}\], \[\text{HF}\] etc. Here, the one-electron density is already at the one end of the molecules. The \[\text{HCl}\] electron density moves from $\text{H}$ to $\text{Cl}$ creating a dipole on molecules that attracts the other molecules from the surrounding towards it. It is shown in the following figure
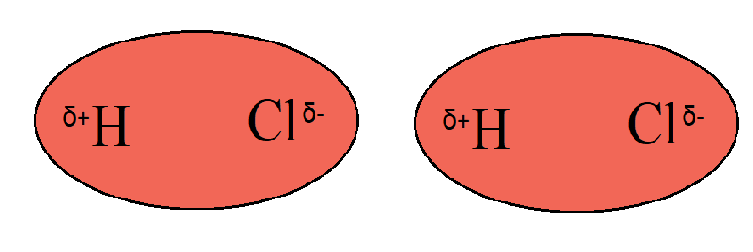
2) Dipole Induced dipoles forces exist between the neutral polar molecules. This is the interaction of a molecule containing permanent dipole moment and with the molecule having no permanent dipole. For example, the non-polar molecule undergoes the separation of charges as another polar molecule approaches towards it.

3) London forces of attraction: The molecules which have the induced dipole can induce the dipole in neighbouring molecules. This forms the dipoles due to the induced dipole interaction. The London force of attraction exists in the gaseous molecules. London-London dispersion forces exist in helium. These are forces between nonpolar molecules. This forces are found in ${{\text{F}}_{\text{2}}}$, ${{\text{I}}_{\text{2}}}$
Van der Waals forces of attraction exist between the inert gases or Noble gases, elementary gases and between the rate gases. Therefore, we can say that the van der Waals force is applicable to a mixture of gases.
Hence, the correct option is (C).
Additional information:
The strength of Van der Waals forces
This forces depend on the size and shape of molecule
1) Size :In periodic tables, the boiling point of Noble gases increases as we move down in groups. This is because the radius of the atoms increases. Therefore larger the atomic radii higher is distance and greater possibility of polarization. Therefore bigger molecules have higher boiling points.
2) Shape of molecules is an important strength of force. Long and thin molecules are capable of developing dipoles than that of the fat and short molecules.
Note: Van der Waals forces are distance dependent forces. The interaction is maximum when the distance between the molecules is less. Gas particles interact with each other thus one of the above forces is always present in inert, rate or elementary gas.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




