
When the value of entropy is greater, then the ability for work is______
A. Maximum
B. Minimum
C. Medium
D. None of these
Answer
233.1k+ views
Hint: To answer this question, we must first know the concept of entropy. This will allow us to figure out the relationship between the entropy of a system and the ability of the system to do work.
Complete Step by Step Solution:
The concept of entropy is one of the fundamental concepts of thermodynamics. This concept came about from the Carnot Engine model.
The Carnot Engine is an engine that converts heat (thermal energy) into mechanical work. A heat engine acts by transferring heat from a hot region to a cold region and, in this process, converting some of the heat into mechanical work, in a cyclic manner. A steam engine using the heat generated by the burning of coal in a furnace to drive the wheels of a train is a good example of a heat engine. A schematic of the Carnot Engine is shown below:
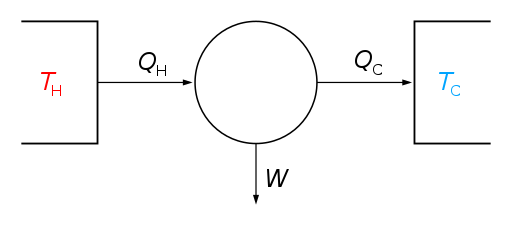
Image: Schematic of the Carnot Engine
The Carnot Engine consists of a “working body” (also called system) which can be any fluid/vapour that can convert the heat introduced to it into mechanical work (W). The system (represented as a circle in the schematic above) takes in heat (\[{Q_H}\]) from a heat source (also called a hot reservoir) which is at a temperature\[{T_H}\]. The coal furnace in a steam engine is an example of a heat source. The system (the steam itself in a steam engine) takes in heat \[{Q_H}\] and converts it into mechanical work W (which drives pistons attached to the wheels of the steam engine, driving the train forward). The excess heat that the system could not convert into work (\[{Q_C}\]) is sent into a heat sink (also called a cold reservoir) which exists at a comparably lower temperature\[{T_C}\]. In a steam engine, the condenser (which is a component that has cold water flowing through it) acts as a heat sink.
Quantitatively, we can see that the input heat \[{Q_H}\] is being converted into mechanical work W and some amount of waste heat \[{Q_C}\] in one cycle. Thus, we can say that
\[{Q_H} = W + {Q_C}\] \[ \Rightarrow W = {Q_H} - {Q_C}\] … (1)
The efficiency of this engine (expressed as a percentage) is defined as\[\eta = \dfrac{W}{{{Q_H}}} \times 100 = \dfrac{{{Q_H} - {Q_C}}}{{{Q_H}}} \times 100\] … (2)
From equation (2), we can see that if the engine were to operate at 100% efficiency, then \[\dfrac{W}{{{Q_H}}} = 1 \Rightarrow W = {Q_H}\] and from equation (1), we can see that \[W = {Q_H}\] if and only if \[{Q_C} = 0\].
Thus, if the engine did not have any heat losses whatsoever, it would convert all the input heat into mechanical work (W) and be 100% efficient. In such circumstances, the work obtained (W) would be maximum.
Such situations are, unfortunately, impossible because any real engine will always have some form of energy loss and will not be 100% efficient. Real engines (such as the steam engine) have moving parts which will produce friction and sound leading to some amount of energy losses and, consequently, a partial loss in the engine’s ability to convert the input heat energy into work.
The inevitable partial loss of an engine’s ability to convert heat energy into work is represented by entropy (S). This description of entropy was one of the first to be formulated. Thus, we can say that if the entropy of a system (such as a heat engine) is high, its ability to perform work will be low.
So, a system having maximum entropy will have minimal ability to perform work.
Therefore, option B is correct.
Note: You might find some sources saying that if the value of entropy is greater (\[\Delta S > 0\] ), the ability of the system to perform work would be maximum since \[\Delta G = \Delta H - T\Delta S\] with \[\Delta S > 0\] would indicate that \[\Delta G < 0\]. This approach would not be correct since \[\Delta S\] represents the change in entropy for a process that the system undergoes, not the absolute entropy of the system itself (which is represented by S). The question here is regarding the absolute entropy of the system.
Complete Step by Step Solution:
The concept of entropy is one of the fundamental concepts of thermodynamics. This concept came about from the Carnot Engine model.
The Carnot Engine is an engine that converts heat (thermal energy) into mechanical work. A heat engine acts by transferring heat from a hot region to a cold region and, in this process, converting some of the heat into mechanical work, in a cyclic manner. A steam engine using the heat generated by the burning of coal in a furnace to drive the wheels of a train is a good example of a heat engine. A schematic of the Carnot Engine is shown below:

Image: Schematic of the Carnot Engine
The Carnot Engine consists of a “working body” (also called system) which can be any fluid/vapour that can convert the heat introduced to it into mechanical work (W). The system (represented as a circle in the schematic above) takes in heat (\[{Q_H}\]) from a heat source (also called a hot reservoir) which is at a temperature\[{T_H}\]. The coal furnace in a steam engine is an example of a heat source. The system (the steam itself in a steam engine) takes in heat \[{Q_H}\] and converts it into mechanical work W (which drives pistons attached to the wheels of the steam engine, driving the train forward). The excess heat that the system could not convert into work (\[{Q_C}\]) is sent into a heat sink (also called a cold reservoir) which exists at a comparably lower temperature\[{T_C}\]. In a steam engine, the condenser (which is a component that has cold water flowing through it) acts as a heat sink.
Quantitatively, we can see that the input heat \[{Q_H}\] is being converted into mechanical work W and some amount of waste heat \[{Q_C}\] in one cycle. Thus, we can say that
\[{Q_H} = W + {Q_C}\] \[ \Rightarrow W = {Q_H} - {Q_C}\] … (1)
The efficiency of this engine (expressed as a percentage) is defined as\[\eta = \dfrac{W}{{{Q_H}}} \times 100 = \dfrac{{{Q_H} - {Q_C}}}{{{Q_H}}} \times 100\] … (2)
From equation (2), we can see that if the engine were to operate at 100% efficiency, then \[\dfrac{W}{{{Q_H}}} = 1 \Rightarrow W = {Q_H}\] and from equation (1), we can see that \[W = {Q_H}\] if and only if \[{Q_C} = 0\].
Thus, if the engine did not have any heat losses whatsoever, it would convert all the input heat into mechanical work (W) and be 100% efficient. In such circumstances, the work obtained (W) would be maximum.
Such situations are, unfortunately, impossible because any real engine will always have some form of energy loss and will not be 100% efficient. Real engines (such as the steam engine) have moving parts which will produce friction and sound leading to some amount of energy losses and, consequently, a partial loss in the engine’s ability to convert the input heat energy into work.
The inevitable partial loss of an engine’s ability to convert heat energy into work is represented by entropy (S). This description of entropy was one of the first to be formulated. Thus, we can say that if the entropy of a system (such as a heat engine) is high, its ability to perform work will be low.
So, a system having maximum entropy will have minimal ability to perform work.
Therefore, option B is correct.
Note: You might find some sources saying that if the value of entropy is greater (\[\Delta S > 0\] ), the ability of the system to perform work would be maximum since \[\Delta G = \Delta H - T\Delta S\] with \[\Delta S > 0\] would indicate that \[\Delta G < 0\]. This approach would not be correct since \[\Delta S\] represents the change in entropy for a process that the system undergoes, not the absolute entropy of the system itself (which is represented by S). The question here is regarding the absolute entropy of the system.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




