
Toluene reacts with an excess of \[C{l_2}\] in presence of sunlight to give a product which on hydrolysis followed by reaction with NaOH gives
A.
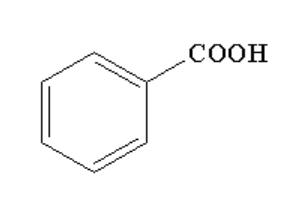
B.
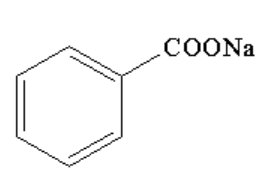
C.
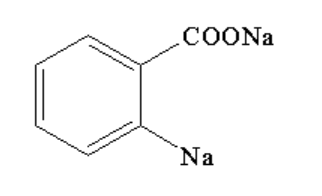
D. None of these
Answer
233.1k+ views
Hint: Toluene is a compound where a methyl group replaces the hydrogen of benzene. The reaction of toluene with chlorine in the presence of sunlight gives a monochlorinated product.
Complete Step by Step Solution:
The chlorination reaction is the one where a chlorine atom is added to the group. The chlorination reaction takes place in the presence of light, so the reaction is a photochemical reaction.
Toluene on reacting with chlorine in presence of sunlight gives a monochlorinated product 1-(chloromethyl)benzene and one mole of hydrochloric acid which on further reaction gives a tri chlorinated product 1-(trichloromethyl)benzene and three moles of hydrochloric acid.
The complete reaction is shown below.
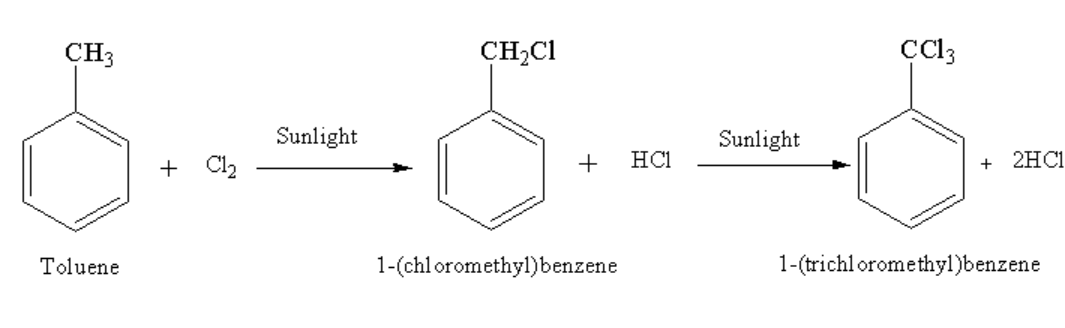
Image: Reaction of toluene with chlorine in presence of sunlight
Further 1-(trichloromethyl)benzene reacting with three moles of sodium hydroxide gives phenylmethanetriol and three moles of sodium chloride. Here, nucleophilic substitution takes place as the leaving group chlorine is replaced by hydroxide nucleophile. As the compound formed is unstable so a water molecule is removed forming a benzoic acid. Benzoic acid further reacts with sodium hydroxide to form sodium benzoate by removing water molecules.
The reaction is shown below:
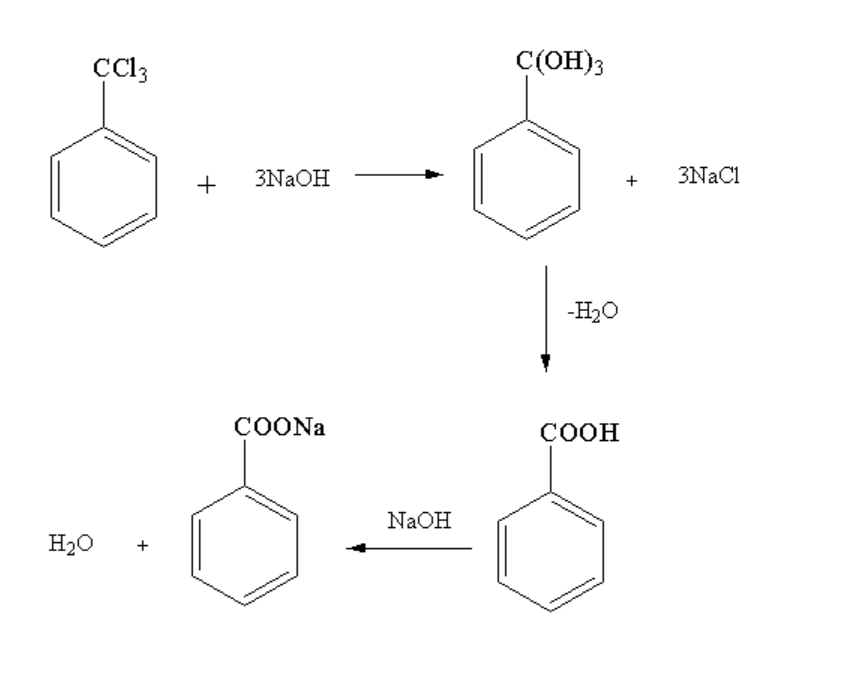
Image: Formation of sodium benzoate
Therefore, the correct option is B.
Note: It should be noted that when toluene undergoes chlorination in presence of iron o-chlorotoluene and p-chlorotoluene are formed. Methyl group shows a +I effect and releases electrons towards the benzene ring therefore the methyl group is ortho and para directing in nature.
Complete Step by Step Solution:
The chlorination reaction is the one where a chlorine atom is added to the group. The chlorination reaction takes place in the presence of light, so the reaction is a photochemical reaction.
Toluene on reacting with chlorine in presence of sunlight gives a monochlorinated product 1-(chloromethyl)benzene and one mole of hydrochloric acid which on further reaction gives a tri chlorinated product 1-(trichloromethyl)benzene and three moles of hydrochloric acid.
The complete reaction is shown below.

Image: Reaction of toluene with chlorine in presence of sunlight
Further 1-(trichloromethyl)benzene reacting with three moles of sodium hydroxide gives phenylmethanetriol and three moles of sodium chloride. Here, nucleophilic substitution takes place as the leaving group chlorine is replaced by hydroxide nucleophile. As the compound formed is unstable so a water molecule is removed forming a benzoic acid. Benzoic acid further reacts with sodium hydroxide to form sodium benzoate by removing water molecules.
The reaction is shown below:

Image: Formation of sodium benzoate
Therefore, the correct option is B.
Note: It should be noted that when toluene undergoes chlorination in presence of iron o-chlorotoluene and p-chlorotoluene are formed. Methyl group shows a +I effect and releases electrons towards the benzene ring therefore the methyl group is ortho and para directing in nature.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




