
Toluene is nitrated, and the resulting product is reduced with tin and hydrochloric acid. The product obtained is diazotized and heated with cuprous bromide. The reaction mixture formed contains :
(A) Mixture of o - and p - bromoanilines
(B) Mixture of o - and m - bromotoulenes
(C) Mixture of o - and p - bromotoulenes
(D) Mixture of o - and p - dibromobenzenes
Answer
233.1k+ views
Hint: Toluene is a transparent, colourless liquid that, when heated to room temperature, turns into a vapour. Toluene is a naturally occurring component of crude oil and is used in the production of paints, lacquers, explosives (TNT), and glues, in addition to oil refining. Toluene exposure can lead to a variety of symptoms, including irritation of the eyes and nose, exhaustion, headache, dilated pupils, tears, anxiety, muscle fatigue, sleeplessness, and nerve damage.
Complete Step by Step Answer:
In the first step, toluene is nitrated with nitric acid ($HN{{O}_{3}}$), it forms o - and p - nitrotoulenes. The nitrotoulenes on reduction with tin and hydrochloric acid ($HCl$) forms o- and p- toulidine. When these toulidines is diazotized and heated with cuprous bromide, the product formed is a mixture of o-and p-bromotoulenes.
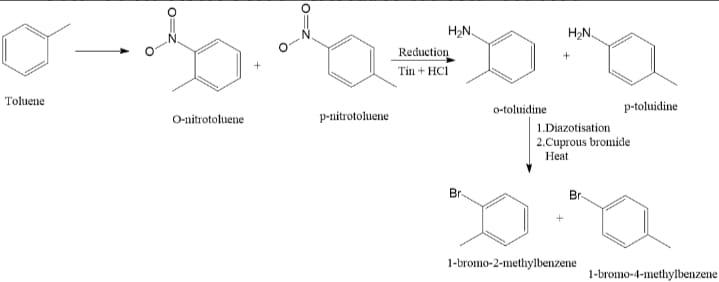
Correct Option: (C) Mixture of o - and p - bromotoulenes.
Note: The methyl group present in toluene is an electron-donating group. The methyl group activates the benzene ring. The electron donating groups are o - and p - directing. On the other hand, if an electron releasing group is attached to a ring, it deactivates the ring.
Complete Step by Step Answer:
In the first step, toluene is nitrated with nitric acid ($HN{{O}_{3}}$), it forms o - and p - nitrotoulenes. The nitrotoulenes on reduction with tin and hydrochloric acid ($HCl$) forms o- and p- toulidine. When these toulidines is diazotized and heated with cuprous bromide, the product formed is a mixture of o-and p-bromotoulenes.

Correct Option: (C) Mixture of o - and p - bromotoulenes.
Note: The methyl group present in toluene is an electron-donating group. The methyl group activates the benzene ring. The electron donating groups are o - and p - directing. On the other hand, if an electron releasing group is attached to a ring, it deactivates the ring.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




