
The total number of possible dichloro derivatives of n-butene are:
(A) 2
(B) 4
(C) 5
(D) 6
Answer
233.1k+ views
Hint: We need to check the position of chlorine with respect to the double bond in the structure of n-butene. As the question is asked about n-butene which means we can place the double bond in three positions with respect to chlorine atoms and no branches will be introduced to the carbon chain. The number of possible structures for n-butene are as follows:

But-1-ene
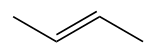
But-2-ene
Complete Step by Step Solution:
n-Butene or normal butene are considered as the stable compounds but the unsaturated carbon-carbon double bonds which consists of $\pi $ electrons make them more reactive due to the attack of electrophile becomes easy due to the presence of cloud of electrons, as compared to similar alkanes as which are more inert compounds in various ways. As per the number of structures of n-butene, the possible constitutional dichloro derivatives of n-butene are as follows:
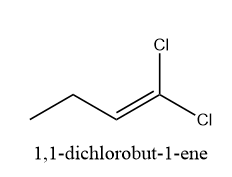
a) 1,1-dichlorobut-1-ene
b) 1,1-dichlorobut-2-ene

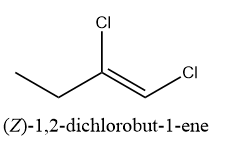
c) 1,2-dichlorobut-1-ene
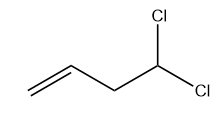
d) 4,4-dichlorobut-1-ene
e) 1,3-dichlorobut-2-ene
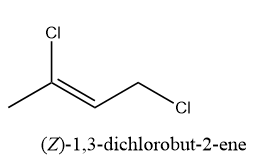
f) 1,3-dichlorobut-1-ene
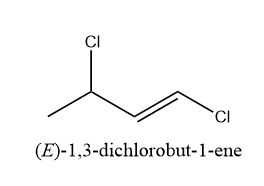
Hence, the total number of possible constitutional dichloro derivatives of n-butene are 6. Therefore, option (D) is the correct answer.
Note: It is important to note that the answer in such questions may vary due to the possibility of stereoisomerism in the compound. So, whenever the question is asked in general, only constitutional isomers of the compound are included. The possible stereoisomers that can be formed in case of alkene are E-Z geometrical isomerism that are similar in molecular structure but differ in arrangement of atoms in space.

But-1-ene

But-2-ene
Complete Step by Step Solution:
n-Butene or normal butene are considered as the stable compounds but the unsaturated carbon-carbon double bonds which consists of $\pi $ electrons make them more reactive due to the attack of electrophile becomes easy due to the presence of cloud of electrons, as compared to similar alkanes as which are more inert compounds in various ways. As per the number of structures of n-butene, the possible constitutional dichloro derivatives of n-butene are as follows:
a) 1,1-dichlorobut-1-ene

b) 1,1-dichlorobut-2-ene

c) 1,2-dichlorobut-1-ene

d) 4,4-dichlorobut-1-ene

e) 1,3-dichlorobut-2-ene

f) 1,3-dichlorobut-1-ene

Hence, the total number of possible constitutional dichloro derivatives of n-butene are 6. Therefore, option (D) is the correct answer.
Note: It is important to note that the answer in such questions may vary due to the possibility of stereoisomerism in the compound. So, whenever the question is asked in general, only constitutional isomers of the compound are included. The possible stereoisomers that can be formed in case of alkene are E-Z geometrical isomerism that are similar in molecular structure but differ in arrangement of atoms in space.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




