
The strongest acid among the following is:
(A) o-methoxyphenol
(B) m-methoxyphenol
(C) p-methoxyphenol
(D) phenol
Answer
233.1k+ views
Hint: The acidity of the derivatives of phenol depends upon the stability of the corresponding phenoxide ion. Presence of EWG on phenyl rings stabilizes the phenoxide ion and presence of EDG destabilizes the phenoxide ion.
Complete step by step solution:
Here, we are being asked to compare the acidic strength of the given phenol derivatives.
-We know that phenol loses the proton from –OH group and forms phenoxide ions. Upon the stability of that phenoxide ion, the stability of the corresponding acid is measured. Let’s see the formation of phenoxide ion and how it is stabilized.
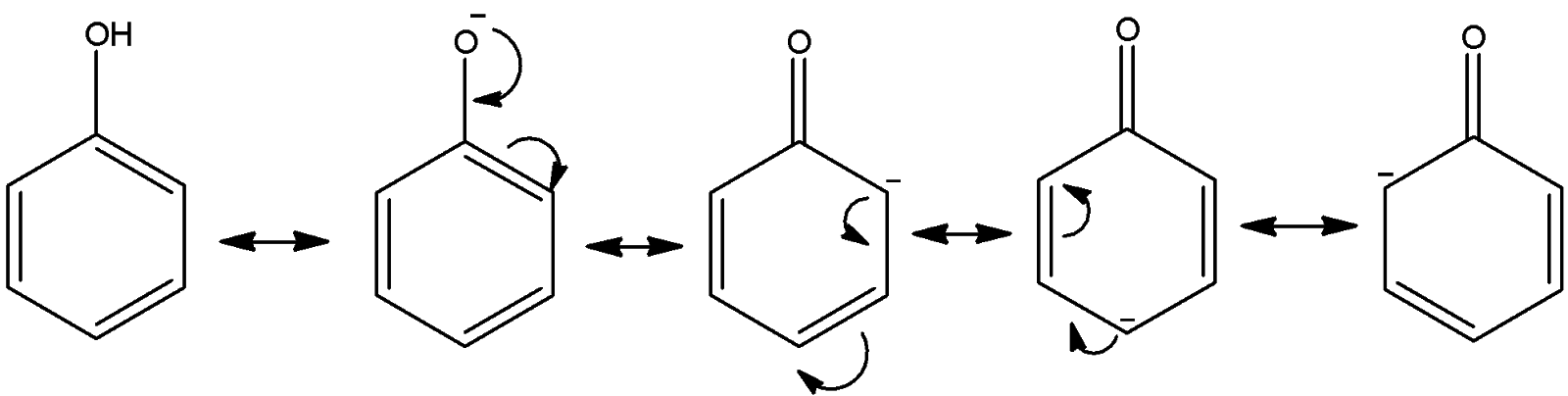
Here, we can see that the negative charge on oxygen is stabilized by resonance. We can see four canonical structures of the phenoxide ion.
-Now, depending upon the substituent groups the acidity of the phenol derivative may differ.
-If there is an electron-withdrawing group (EWG) attached to the phenyl ring, then the acidity of the corresponding phenol increases and if any electron releasing group (EDG) is present at the phenyl ring, then the acidity of the corresponding phenol decreases.
-So, we can say that if we substitute methoxy group which is an electron-donating group, then it will decrease the acidity of the corresponding phenol because the negative charge is delocalized and the methoxy group being electron donating in nature will destabilize the phenoxide ion and as a result, the acidity of the corresponding phenol will be decreased.
-Thus, we can conclude that acidity of ortho, meta and para derivatives of phenol will be less than phenol.
So, the correct answer to this question is (D).
Note: The effect of the presence of substituent group at ortho and para positions have a more strong effect than at meta position. Do not forget that an alkoxy group is an electron-donating group.
Complete step by step solution:
Here, we are being asked to compare the acidic strength of the given phenol derivatives.
-We know that phenol loses the proton from –OH group and forms phenoxide ions. Upon the stability of that phenoxide ion, the stability of the corresponding acid is measured. Let’s see the formation of phenoxide ion and how it is stabilized.

Here, we can see that the negative charge on oxygen is stabilized by resonance. We can see four canonical structures of the phenoxide ion.
-Now, depending upon the substituent groups the acidity of the phenol derivative may differ.
-If there is an electron-withdrawing group (EWG) attached to the phenyl ring, then the acidity of the corresponding phenol increases and if any electron releasing group (EDG) is present at the phenyl ring, then the acidity of the corresponding phenol decreases.
-So, we can say that if we substitute methoxy group which is an electron-donating group, then it will decrease the acidity of the corresponding phenol because the negative charge is delocalized and the methoxy group being electron donating in nature will destabilize the phenoxide ion and as a result, the acidity of the corresponding phenol will be decreased.
-Thus, we can conclude that acidity of ortho, meta and para derivatives of phenol will be less than phenol.
So, the correct answer to this question is (D).
Note: The effect of the presence of substituent group at ortho and para positions have a more strong effect than at meta position. Do not forget that an alkoxy group is an electron-donating group.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




