
The reactivities of methyl chloride, propyl chloride and chlorobenzene are in the order:
A. Methyl chloride > propyl chloride > chlorobenzene
B. Propyl chloride > methyl chloride > chlorobenzene
C. Methyl chloride > chlorobenzene > propyl chloride
D. Chlorobenzene > propyl chloride > methyl chloride
Answer
239.4k+ views
Hint: Reactivities of these alkyl halides towards \[{S_N}2\] reactions depend on the bulkiness of the substituents attached to the chlorine-carrying carbon atom. Resonance also plays a role in governing their reactivity.
Complete Step by Step Solution:
We are required to arrange the reactivities of methyl chloride, propyl chloride and chlorobenzene in the order of their reactivity.
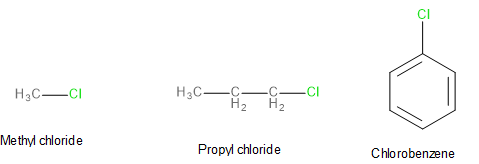
Image: Structures of methyl chloride, propyl chloride and chlorobenzene
Although the order of reactivity would differ based on the reaction in question, since the reaction is not explicitly mentioned here, let us assume that the reaction in question is an \[{S_N}2\] reaction.
The \[{S_N}2\] reaction is a bimolecular, nucleophilic substitution reaction in which a nucleophile (an electron-rich species) attacks the substrate on the chlorine-carrying carbon atom from its “back-side” i.e., the side opposite to/away from the chlorine atom which leads to the formation of a transition state.
The factor governing the reactivity of a molecule towards an\[{S_N}2\]reaction is the steric effect. The steric effect is the effect on the rate of the reaction by the space-filling properties of those parts of a molecule attached to the reacting site. In SN2 reactions the reacting site is the carbon carrying the chlorine atom. The incoming nucleophile must get to a distance within the bonding range of that carbon atom. Therefore, bulky substituents on or near that carbon atom can inhibit the SN2 reaction by preventing the nucleophile from getting close.
The \[{S_N}2\] reaction is fastest in methyl chloride because the chlorine-carrying carbon has only 3 small hydrogens attached to it which do not offer any steric hindrance to any incoming nucleophiles.
Propyl chloride reacts comparatively slower because the chlorine-carrying carbon has two hydrogen atoms as well as a methyl group attached to it which is bulkier and inhibits the \[{S_N}2\] reaction more.
Chlorobenzene has a very low reactivity towards all nucleophilic substitution reactions because the chlorine atom cannot leave the molecule easily. This is because the carbon-chlorine bond has a partial double-bond character due to resonance making it difficult to break.
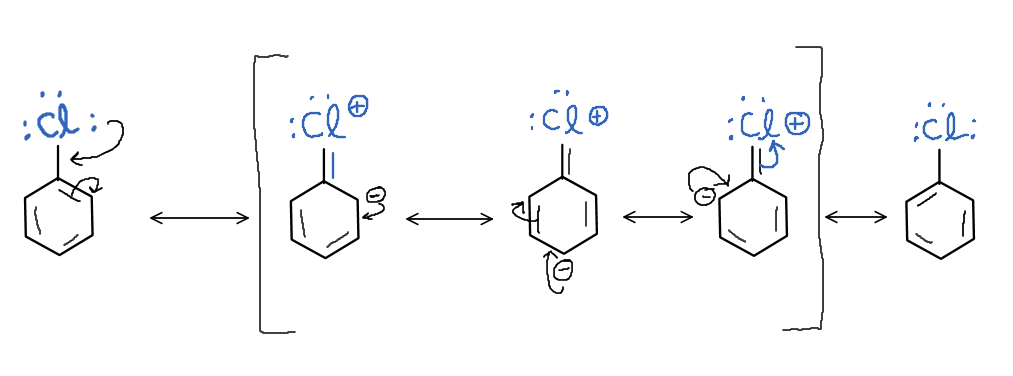
Image: Resonance structures of chlorobenzene.
Thus, the correct order of reactivity would be option A.
Note: In the case of\[{S_N}1\]reaction, methyl chloride would react slower than propyl chloride. This is because the rates of \[{S_N}1\] reactions are determined by the stability of the carbocation intermediates formed. Carbocation stability is determined by hyperconjugative and inductive effects. The degree of hyperconjugation depends directly on the number of the alpha-hydrogens present. Propyl carbocation has two alpha-hydrogens whereas methyl carbocation has none thus, hyperconjugation will be higher in propyl carbocation. The correct option, in this case, would be option B.
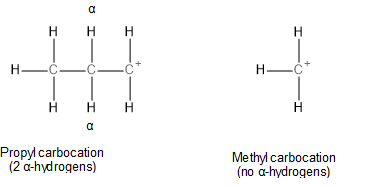
Image: Structures of propyl and methyl carbocations
Complete Step by Step Solution:
We are required to arrange the reactivities of methyl chloride, propyl chloride and chlorobenzene in the order of their reactivity.

Image: Structures of methyl chloride, propyl chloride and chlorobenzene
Although the order of reactivity would differ based on the reaction in question, since the reaction is not explicitly mentioned here, let us assume that the reaction in question is an \[{S_N}2\] reaction.
The \[{S_N}2\] reaction is a bimolecular, nucleophilic substitution reaction in which a nucleophile (an electron-rich species) attacks the substrate on the chlorine-carrying carbon atom from its “back-side” i.e., the side opposite to/away from the chlorine atom which leads to the formation of a transition state.
The factor governing the reactivity of a molecule towards an\[{S_N}2\]reaction is the steric effect. The steric effect is the effect on the rate of the reaction by the space-filling properties of those parts of a molecule attached to the reacting site. In SN2 reactions the reacting site is the carbon carrying the chlorine atom. The incoming nucleophile must get to a distance within the bonding range of that carbon atom. Therefore, bulky substituents on or near that carbon atom can inhibit the SN2 reaction by preventing the nucleophile from getting close.
The \[{S_N}2\] reaction is fastest in methyl chloride because the chlorine-carrying carbon has only 3 small hydrogens attached to it which do not offer any steric hindrance to any incoming nucleophiles.
Propyl chloride reacts comparatively slower because the chlorine-carrying carbon has two hydrogen atoms as well as a methyl group attached to it which is bulkier and inhibits the \[{S_N}2\] reaction more.
Chlorobenzene has a very low reactivity towards all nucleophilic substitution reactions because the chlorine atom cannot leave the molecule easily. This is because the carbon-chlorine bond has a partial double-bond character due to resonance making it difficult to break.

Image: Resonance structures of chlorobenzene.
Thus, the correct order of reactivity would be option A.
Note: In the case of\[{S_N}1\]reaction, methyl chloride would react slower than propyl chloride. This is because the rates of \[{S_N}1\] reactions are determined by the stability of the carbocation intermediates formed. Carbocation stability is determined by hyperconjugative and inductive effects. The degree of hyperconjugation depends directly on the number of the alpha-hydrogens present. Propyl carbocation has two alpha-hydrogens whereas methyl carbocation has none thus, hyperconjugation will be higher in propyl carbocation. The correct option, in this case, would be option B.

Image: Structures of propyl and methyl carbocations
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding the Angle of Deviation in a Prism

Understanding Electromagnetic Waves and Their Importance

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules - 2025-26




