
The product ‘C’ in the following sequence of a chemical reaction is :
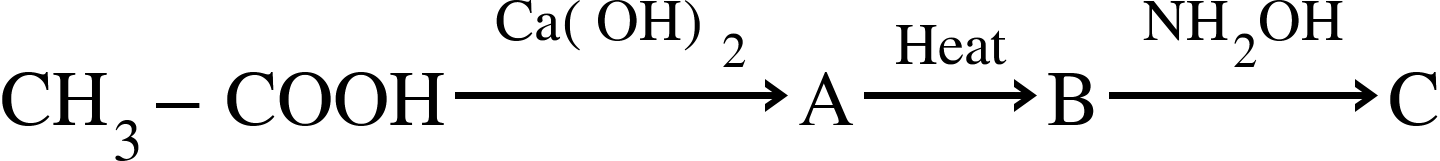
(A) Acetaldoxime
(B) Formaldoxime
(C) Ethane Nitrile
(D) Acetoxime
Answer
233.1k+ views
Hint:Chemical reaction is defined as the process which leads to the transformation of chemical substances from one form to another. In a chemical reaction, the substances undergoing transformation are called reactants and the new substances that are formed are called products. Oximes are the chemical compounds that belong to the class of imines, and they have the general formula of ${ R }_{ 1 }{ R }_{ 2 }{ C=N-OH }$
Complete step by step solution:
I) When acetic acid reacts with calcium hydroxide it gives calcium acetate as the product.
II) When calcium acetate is heated or dry distillates it gives acetone.
III) Acetone undergoes a condensation reaction with hydroxylamine it gives acetoxime.
> The following reaction will take place;
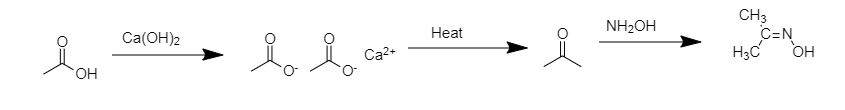
Hence, the correct option is D.
> Heating of solid materials to produce gaseous products (which may condense into liquids or solids) is called dry distillation.
- The method has been used to obtain liquid fuels from coal and wood. This method usually requires higher temperatures than classical distillation.
- Condensation reaction means two reactants react with the elimination of small molecules such as water, hydrochloric acid, etc.
- Oximes are the chemical compounds that belong to the class of imines, having the general formula of ${ R }_{ 1 }{ R }_{ 2 }{ C=N-OH }$, here ${ R }_{ 1 }$ is the organic side-chain whereas ${ R }_{ 2 }$ is the hydrogen, which forms an aldoxime, or like another group of organic compounds such as ketoxime.
Note: The possibility to make a mistake is that you may choose option A. But there is a difference between acetaldoxime and acetoxime. Acetaldoxime is one of the simplest oxime-containing compounds while acetoxime is one of the simplest forms of ketoxime.
Complete step by step solution:
I) When acetic acid reacts with calcium hydroxide it gives calcium acetate as the product.
II) When calcium acetate is heated or dry distillates it gives acetone.
III) Acetone undergoes a condensation reaction with hydroxylamine it gives acetoxime.
> The following reaction will take place;
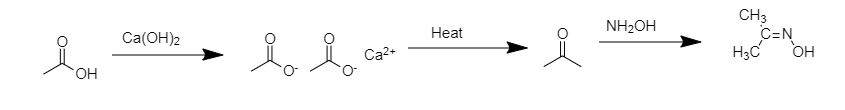
Hence, the correct option is D.
> Heating of solid materials to produce gaseous products (which may condense into liquids or solids) is called dry distillation.
- The method has been used to obtain liquid fuels from coal and wood. This method usually requires higher temperatures than classical distillation.
- Condensation reaction means two reactants react with the elimination of small molecules such as water, hydrochloric acid, etc.
- Oximes are the chemical compounds that belong to the class of imines, having the general formula of ${ R }_{ 1 }{ R }_{ 2 }{ C=N-OH }$, here ${ R }_{ 1 }$ is the organic side-chain whereas ${ R }_{ 2 }$ is the hydrogen, which forms an aldoxime, or like another group of organic compounds such as ketoxime.
Note: The possibility to make a mistake is that you may choose option A. But there is a difference between acetaldoxime and acetoxime. Acetaldoxime is one of the simplest oxime-containing compounds while acetoxime is one of the simplest forms of ketoxime.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




