
The presence of delocalized π electrons in benzene indicates that it is:
(A) Less stable than cyclohexatriene
(B) More stable than cyclohexatriene
(C) More basic than cyclohexatriene
(D) Both (b) & (c)
Answer
233.1k+ views
Hint: (1) Benzene is an aromatic compound.
(2) Benzene is not an alkene because unlike alkenes, benzene does not undergo additional reactions easily.
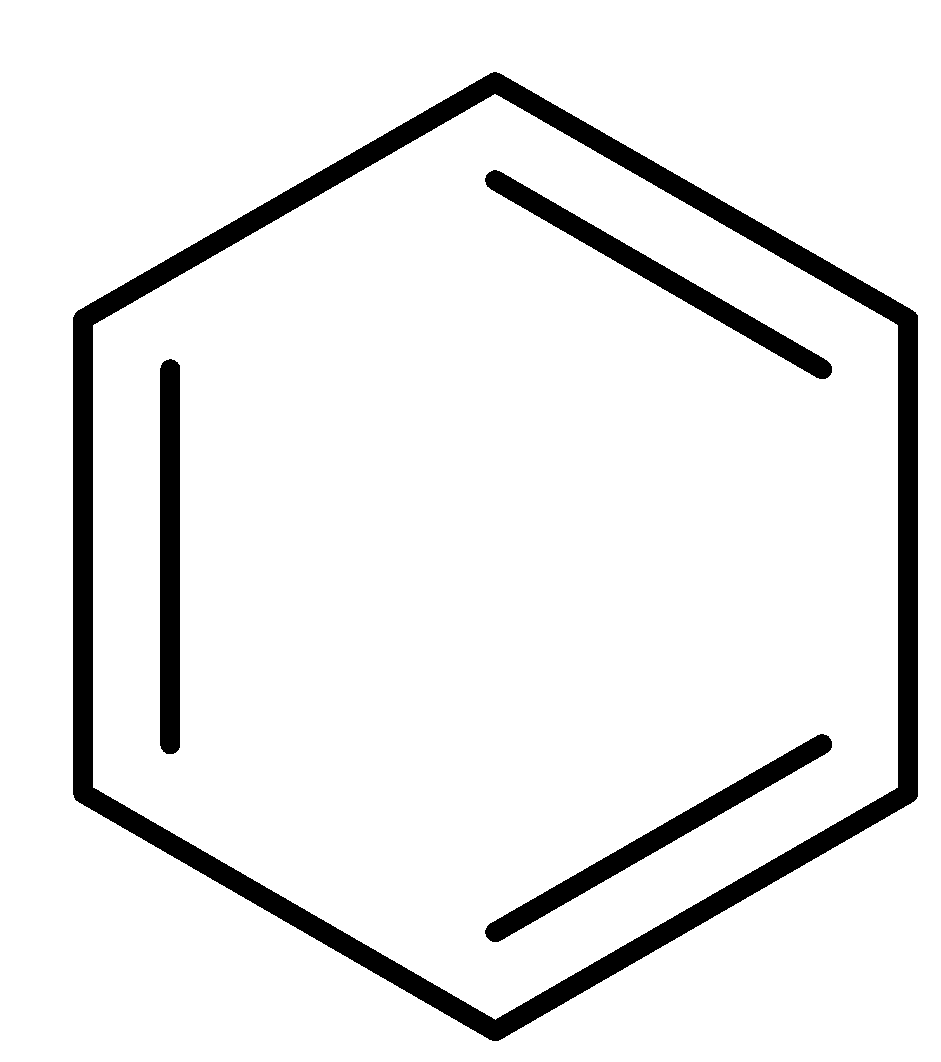
Complete step-by-step answer: By delocalized pi bond, we mean that the electrons in the pi bond are free to move over more than two nuclei. The molecular formula of benzene is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\] . The structure of benzene is:
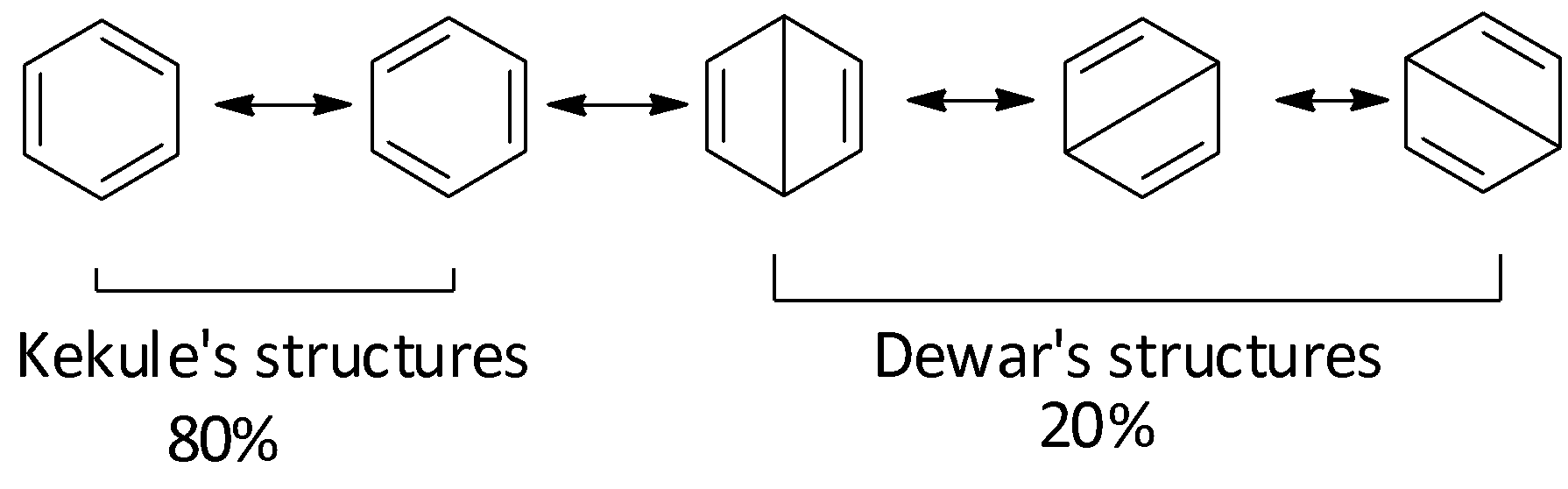
Benzene is considered to be a resonance hybrid of the following structures:
Each carbon in a benzene molecule is ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized. The three ${\text{s}}{{\text{p}}^{\text{2}}}$ hybrid orbitals are utilized in the formation of three sigma bonds which leads to a planar hexagonal structure. Now, one unhybridized 2p orbital is left in each carbon atom. Since there are six carbons in benzene, there will be a total of six partially filled unhybridized 2p orbitals. These forms of 3 pi bonds by sideways overlap. Each of these p orbitals can also simultaneously overlap with other p-orbitals on both sides forming a delocalized pi-electron cloud which lies above and below the plane of the whole ring.
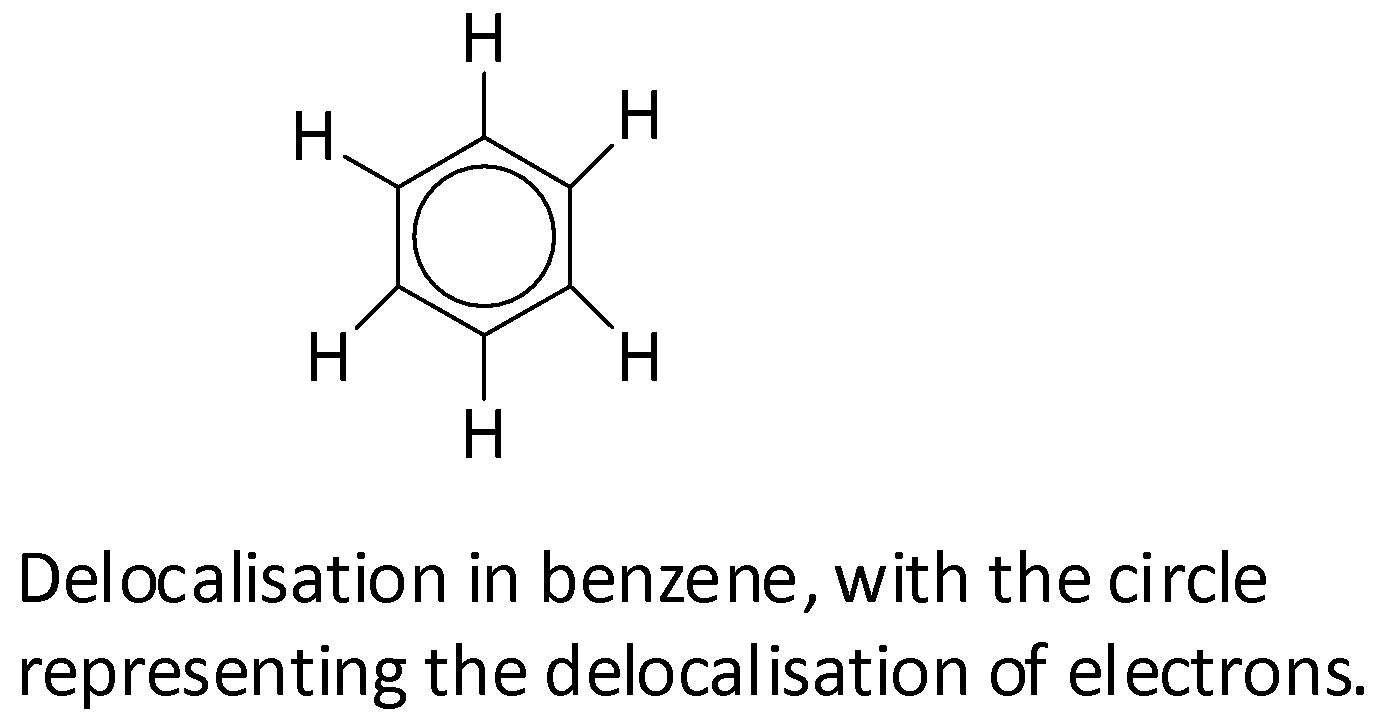
Extra stability is provided to the benzene molecule by this delocalization of pi-electrons. Any factor which tries to eliminate this delocalization will decrease the stability of the benzene molecule. This is why benzene does not undergo addition reactions like alkenes because addition reactions lead to the elimination of the delocalization. Resonance in benzene also supports that all the bond lengths of all the six carbon-carbon bonds are equivalent and intermediate between those of ${\text{C - C}}$ single bonds and ${\text{C = C}}$ double bonds.
On the other hand, cyclohexatriene is a molecule without any resonance or delocalization. So it is not stable and cannot exist.
Thus, benzene is more stable than cyclohexatriene due to delocalization of pi-electrons. So, option (b) is correct.
Note: Delocalization of pi-electrons in a molecule results in the attainment of extra stability by that molecule in comparison to those molecules which lack in delocalization.
(2) Benzene is not an alkene because unlike alkenes, benzene does not undergo additional reactions easily.
Complete step-by-step answer: By delocalized pi bond, we mean that the electrons in the pi bond are free to move over more than two nuclei. The molecular formula of benzene is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\] . The structure of benzene is:

Benzene is considered to be a resonance hybrid of the following structures:

Each carbon in a benzene molecule is ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized. The three ${\text{s}}{{\text{p}}^{\text{2}}}$ hybrid orbitals are utilized in the formation of three sigma bonds which leads to a planar hexagonal structure. Now, one unhybridized 2p orbital is left in each carbon atom. Since there are six carbons in benzene, there will be a total of six partially filled unhybridized 2p orbitals. These forms of 3 pi bonds by sideways overlap. Each of these p orbitals can also simultaneously overlap with other p-orbitals on both sides forming a delocalized pi-electron cloud which lies above and below the plane of the whole ring.

Extra stability is provided to the benzene molecule by this delocalization of pi-electrons. Any factor which tries to eliminate this delocalization will decrease the stability of the benzene molecule. This is why benzene does not undergo addition reactions like alkenes because addition reactions lead to the elimination of the delocalization. Resonance in benzene also supports that all the bond lengths of all the six carbon-carbon bonds are equivalent and intermediate between those of ${\text{C - C}}$ single bonds and ${\text{C = C}}$ double bonds.
On the other hand, cyclohexatriene is a molecule without any resonance or delocalization. So it is not stable and cannot exist.
Thus, benzene is more stable than cyclohexatriene due to delocalization of pi-electrons. So, option (b) is correct.
Note: Delocalization of pi-electrons in a molecule results in the attainment of extra stability by that molecule in comparison to those molecules which lack in delocalization.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




