
The preparation of benzene from acetylene can also be said as:
A. Oxidation
B. Polymerization
C. Dehydration
D. Condensation
Answer
233.1k+ views
Hint: Acetylene is a simplest alkyne with chemical formula \[{C_2}{H_2}\]. Chemical formula of benzene is \[{C_6}{H_6}\]. So, if we take 3 molecules of ethyne or acetylene and react under further conditions we get the benzene. Now let us see the complete step by step procedure to get the benzene from acetylene.
Complete step by step solution:
- Acetylene has the formula as \[{C_2}{H_2}\] while benzene has a chemical formula as \[{C_6}{H_6}\].
- Benzene is an organic chemical compound that is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each other.
- We can see that if we take 3 molecules of acetylene and allow them to react under certain conditions we can easily get benzene. Now we will see how to proceed to get the final result.
- Pass the three molecules of ethyne through the red hot Fe tube at 873K. All the three molecules of ethyne undergo cyclic polymerization to form benzene. We can also say that all the three molecules of ethyne undergo condensation or trimerization to form benzene. This method is also called polymerization.
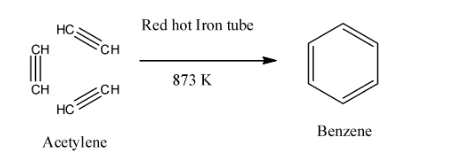
Here, we can see that all the three molecules of ethyne undergo cyclic polymerization to form benzene.
So, from above we can conclude that benzene can be formed from acetylene by the process of polymerization.
Note: Remember that apart from the above listed process benzene can also be prepared by aromatic acids through decarboxylation reaction. We can also prepare benzene from phenol as well through diazonium salt intermediate.
Complete step by step solution:
- Acetylene has the formula as \[{C_2}{H_2}\] while benzene has a chemical formula as \[{C_6}{H_6}\].
- Benzene is an organic chemical compound that is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each other.
- We can see that if we take 3 molecules of acetylene and allow them to react under certain conditions we can easily get benzene. Now we will see how to proceed to get the final result.
- Pass the three molecules of ethyne through the red hot Fe tube at 873K. All the three molecules of ethyne undergo cyclic polymerization to form benzene. We can also say that all the three molecules of ethyne undergo condensation or trimerization to form benzene. This method is also called polymerization.

Here, we can see that all the three molecules of ethyne undergo cyclic polymerization to form benzene.
So, from above we can conclude that benzene can be formed from acetylene by the process of polymerization.
Note: Remember that apart from the above listed process benzene can also be prepared by aromatic acids through decarboxylation reaction. We can also prepare benzene from phenol as well through diazonium salt intermediate.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
Understanding Average and RMS Value in Electrical Circuits

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

Understanding Elastic Collisions in Two Dimensions

For pure water A pH increases while pOH decreases with class 11 chemistry JEE_Main

Which of the following is most stable A Sn2+ B Ge2+ class 11 chemistry JEE_Main

Other Pages
NCERT Solutions For Class 11 Chemistry in Hindi Chapter 8 Redox Reactions (2025-26)

An ideal gas is at pressure P and temperature T in class 11 chemistry JEE_Main

In Carius method of estimation of halogens 015g of class 11 chemistry JEE_Main

Understanding Collisions: Types and Examples for Students

NCERT Solutions For Class 11 Chemistry in Hindi Chapter 1 Some Basic Concepts of Chemistry (2025-26)

Happy New Year Wishes 2026 – 100+ Messages, Quotes, Shayari, Images & Status in All Languages




