
The number of peroxide bonds in perxenate ion \[{{[Xe{{O}_{6}}]}^{-4}}\] is:
(A) 0
(B) 2
(C) 3
(D) 1
Answer
233.1k+ views
Hint: Peroxide bond means, a compound in which two oxygen atoms are attached together by a single bond. A number of organic and inorganic peroxides are available. The compounds that have a peroxide bond are called as peroxides.
Complete step by step answer:
Now we have to find the number of peroxide bond present in the given molecule perxenate ion is \[{{[Xe{{O}_{6}}]}^{-4}}\].
The structure of the \[{{[Xe{{O}_{6}}]}^{-4}}\]is as follows.
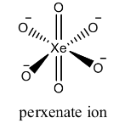
Perxenate ion has six oxygen atoms and is connected to central metal xenon atom. The structure of perxenate ions is regular octahedral.
Two oxygen atoms are attached to xenon atoms through double bonds and the remaining four hydrogen atoms are attached to xenon through single bonds.
But we cannot see any peroxide bonds in the above structure.
Therefore the number of peroxide bonds in the given molecule is zero.
So, the correct option is A.
Additional information:
Peroxides can be used as bleaching agents, and as initiators in the polymerization reactions to prepare polymers.
Peroxides are used in the preparation of hydrogen peroxide and other oxygen compounds.
\[{{[Xe{{O}_{6}}]}^{-4}}\]can be used to separate minute amounts of americium from curium.
Maximum metal perxenates are stable excluding silver perxenate.
Note: Perxenic acid also called as perxenate ion (\[{{[Xe{{O}_{6}}]}^{-4}}\]). It is a strong oxidizing agent and it is capable of oxidizing silver (I) to silver (III), and copper (II) to copper (III). The perxenate ion is unstable in acidic solutions and very rapidly reduced to\[{{[HXe{{O}_{6}}]}^{-}}\].
Complete step by step answer:
Now we have to find the number of peroxide bond present in the given molecule perxenate ion is \[{{[Xe{{O}_{6}}]}^{-4}}\].
The structure of the \[{{[Xe{{O}_{6}}]}^{-4}}\]is as follows.

Perxenate ion has six oxygen atoms and is connected to central metal xenon atom. The structure of perxenate ions is regular octahedral.
Two oxygen atoms are attached to xenon atoms through double bonds and the remaining four hydrogen atoms are attached to xenon through single bonds.
But we cannot see any peroxide bonds in the above structure.
Therefore the number of peroxide bonds in the given molecule is zero.
So, the correct option is A.
Additional information:
Peroxides can be used as bleaching agents, and as initiators in the polymerization reactions to prepare polymers.
Peroxides are used in the preparation of hydrogen peroxide and other oxygen compounds.
\[{{[Xe{{O}_{6}}]}^{-4}}\]can be used to separate minute amounts of americium from curium.
Maximum metal perxenates are stable excluding silver perxenate.
Note: Perxenic acid also called as perxenate ion (\[{{[Xe{{O}_{6}}]}^{-4}}\]). It is a strong oxidizing agent and it is capable of oxidizing silver (I) to silver (III), and copper (II) to copper (III). The perxenate ion is unstable in acidic solutions and very rapidly reduced to\[{{[HXe{{O}_{6}}]}^{-}}\].
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




