
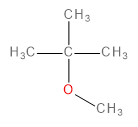
The major product formed in the following reaction is
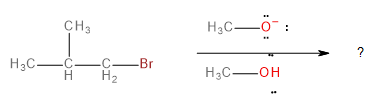
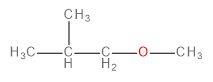
A.
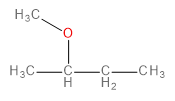
B.
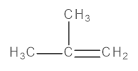
C.
D.
Answer
233.1k+ views
Hint: Here we have an alkyl halide reacting with methoxide ion (\[C{H_3}{O^ - }\] ) in the presence of methanol (\[C{H_3}OH\]). The alkyl halide undergoes an elimination reaction called dehydrohalogenation.
Complete Step by Step Solution:
In this question, we have to predict the product that will be formed when 1-bromo-2-methylpropane reacts with a methoxide ion (\[C{H_3}{O^ - }\] ) in the presence of methanol (\[C{H_3}OH\]).
The methoxide ion is an electron-rich species having lone pairs on the oxygen atom. The carbon-bromine (\[C - Br\] ) bond in 1-bromo-2-methylpropane is polarised towards bromine because of its higher electronegativity. This makes the carbon atom a bit deficient in electrons. Therefore, one might think that the methoxide ion would substitute the bromine in a nucleophilic substitution reaction to form an ether as the final product.
However, this does not happen because the methoxide ion does not act as a nucleophile. Instead, it prefers to attack a hydrogen atom on 1-bromo-2-methylpropane and abstracts it as a proton (\[{H^ + }\]). Thus, the methoxide ion acts as a base.
When the methoxide ion acts as a base, it initiates an elimination reaction instead of a substitution reaction.
Although elimination reactions can occur via multiple mechanisms, in this case, the mechanism followed is a “bimolecular” elimination mechanism, also known as the E2 mechanism.
This is a single-step mechanism involving the abstraction of a beta-hydrogen as a proton and the elimination of bromine as a bromide ion (\[B{r^ - }\]) to form an alkene as the final, major product.
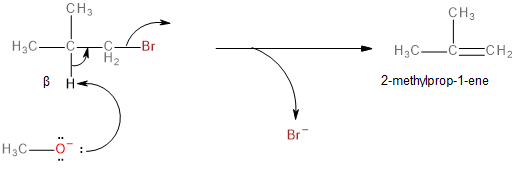
Image: Elimination reaction of 1-bromo-2-methylpropane to form 2-methylprop-1-ene
Thus, option C is correct.
Note: Nucleophilicity and basicity are not the same. Nucleophilicity refers to the ability of an electron-rich species to donate its lone pairs of electrons to electron-deficient species. Therefore, all nucleophiles are Lewis bases. But basicity refers to the ability of an electron-rich species to accept a proton. When an electron-rich species acts as an electron donor (i.e., a nucleophile), it causes substitution. When an electron-rich species acts as a proton acceptor (i.e., a base), it causes elimination. It is highly advised that students learn about the extent of nucleophilicities and basicities of different electron-rich species.
Complete Step by Step Solution:
In this question, we have to predict the product that will be formed when 1-bromo-2-methylpropane reacts with a methoxide ion (\[C{H_3}{O^ - }\] ) in the presence of methanol (\[C{H_3}OH\]).
The methoxide ion is an electron-rich species having lone pairs on the oxygen atom. The carbon-bromine (\[C - Br\] ) bond in 1-bromo-2-methylpropane is polarised towards bromine because of its higher electronegativity. This makes the carbon atom a bit deficient in electrons. Therefore, one might think that the methoxide ion would substitute the bromine in a nucleophilic substitution reaction to form an ether as the final product.
However, this does not happen because the methoxide ion does not act as a nucleophile. Instead, it prefers to attack a hydrogen atom on 1-bromo-2-methylpropane and abstracts it as a proton (\[{H^ + }\]). Thus, the methoxide ion acts as a base.
When the methoxide ion acts as a base, it initiates an elimination reaction instead of a substitution reaction.
Although elimination reactions can occur via multiple mechanisms, in this case, the mechanism followed is a “bimolecular” elimination mechanism, also known as the E2 mechanism.
This is a single-step mechanism involving the abstraction of a beta-hydrogen as a proton and the elimination of bromine as a bromide ion (\[B{r^ - }\]) to form an alkene as the final, major product.

Image: Elimination reaction of 1-bromo-2-methylpropane to form 2-methylprop-1-ene
Thus, option C is correct.
Note: Nucleophilicity and basicity are not the same. Nucleophilicity refers to the ability of an electron-rich species to donate its lone pairs of electrons to electron-deficient species. Therefore, all nucleophiles are Lewis bases. But basicity refers to the ability of an electron-rich species to accept a proton. When an electron-rich species acts as an electron donor (i.e., a nucleophile), it causes substitution. When an electron-rich species acts as a proton acceptor (i.e., a base), it causes elimination. It is highly advised that students learn about the extent of nucleophilicities and basicities of different electron-rich species.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




