
The hybridization state of the central atom in ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ is:
A. $s{p^3}d$
B. $s{p^3}{d^2}$
C. $s{p^3}$
D. ${d^2}s{p^3}$
Answer
233.1k+ views
Hint: Phosphorus pentachloride is the commonly abbreviated as ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$. ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ has a trigonal bipyramidal structure.
Complete step by step answer:
We know that, in phosphorus pentachloride, the phosphorus is the central atom. The electronic configurations of phosphorus and chloride atoms is as follows:
Phosphorous- $\left( {{\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{3}}}} \right)$ and chloride- $\left( {{\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^5}} \right)$
So, one of the “s”, three of the “p” and of the “d” orbitals participate to give a ${\rm{3}}{{\rm{p}}_{\rm{z}}}$ hybridisation. The five orbitals of the ${\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}$ hybrid is occupies singly, so it overlaps with the ${\rm{3}}{{\rm{p}}_{\rm{z}}}$ orbital of the chlorine atom forming five sigma P-Cl bonds. This gives rise to the trigonal bipyramidal shape of the ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ molecule having the bond angles of ${90^\circ }$ and ${120^\circ }$.
We can draw the structure of ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ as follows:
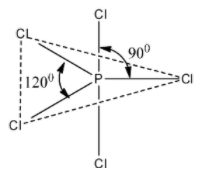
The above ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ structure that we have drawn is a trigonal bipyramidal structure. Due to the trigonal bipyramidal structure, the two ${\rm{C}}{{\rm{l}}^ - }$ atoms of ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ molecule are stretched and lie along the axis and it becomes little longer and so these bonds called as axial bonds, while the other three ${\rm{C}}{{\rm{l}}^ - }$ atoms lie on equator and these bonds are called as equatorial bonds.
So, out of the given four options, A is the correct option.
Note:
Students may get confused in interpreting the structure of the ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$. It should be noted that in ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$, two ${\rm{P}} - {\rm{Cl}}$ bonds remains as axial bonds along the axis and the other three ${\rm{P}} - {\rm{Cl}}$ bonds remains as equatorial bonds.
Complete step by step answer:
We know that, in phosphorus pentachloride, the phosphorus is the central atom. The electronic configurations of phosphorus and chloride atoms is as follows:
Phosphorous- $\left( {{\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{3}}}} \right)$ and chloride- $\left( {{\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^5}} \right)$
So, one of the “s”, three of the “p” and of the “d” orbitals participate to give a ${\rm{3}}{{\rm{p}}_{\rm{z}}}$ hybridisation. The five orbitals of the ${\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}$ hybrid is occupies singly, so it overlaps with the ${\rm{3}}{{\rm{p}}_{\rm{z}}}$ orbital of the chlorine atom forming five sigma P-Cl bonds. This gives rise to the trigonal bipyramidal shape of the ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ molecule having the bond angles of ${90^\circ }$ and ${120^\circ }$.
We can draw the structure of ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ as follows:

The above ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ structure that we have drawn is a trigonal bipyramidal structure. Due to the trigonal bipyramidal structure, the two ${\rm{C}}{{\rm{l}}^ - }$ atoms of ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ molecule are stretched and lie along the axis and it becomes little longer and so these bonds called as axial bonds, while the other three ${\rm{C}}{{\rm{l}}^ - }$ atoms lie on equator and these bonds are called as equatorial bonds.
So, out of the given four options, A is the correct option.
Note:
Students may get confused in interpreting the structure of the ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$. It should be noted that in ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$, two ${\rm{P}} - {\rm{Cl}}$ bonds remains as axial bonds along the axis and the other three ${\rm{P}} - {\rm{Cl}}$ bonds remains as equatorial bonds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




