
The hybridisation in $I{F_7}$ molecule is :
A. $s{p^3}$
B. $s{p^3}{d^2}$
C. $s{p^3}d$
D. $s{p^3}{d^3}$
Answer
233.1k+ views
Hint: In the process of hybridization two or more than two atomic orbital goes in inter-mixing of the atomic orbital and gives hybridized orbital which remains same in the number and energy.
Complete step by step solution:
> The given compound is known as iodine heptafluoride. It has seven bond pairs and one lone pair. In the following diagram pentagonal bipyramidal geometry of molecules is shown . > There are two fluorine atoms above and below the plane which make two pyramids with the plane while five fluorine atoms are arranged in a pentagon in the plane. The geometry of molecules used to predict with VSEPR theory (valence shell electron repulsion theory).In this theory the number of electron pairs are used to predict the geometry of molecules.
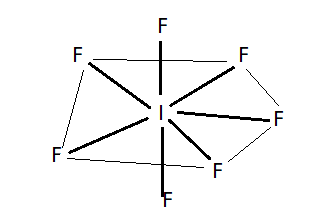
> In $I{F_7}$ molecule ,central metal atom is $I$. The electronic configuration of iodine atom is given below ;
${I_{53}} = 1{s^2},2{s^2},2{p^6},3{s^2},3{p^6},4{s^2},3{d^{10}},4{p^6},5{s^2},4{d^{10}},5{p^5}$ so here is seven atomic orbital that is one s orbitals, three p orbitals and three d orbitals which hybridizes to form seven hybrid orbitals. The geometry of $I{F_7}$ is pentagonal bipyramidal. Seven hybrid $s{p^3}{d^3}$ orbitals are directed towards the corners of a pentagonal bipyramid. Five of the hybrid orbitals are directed towards the corners of a regular pentagon whereas the remaining two are directed above and below the plane. So here option D is correct.
Note : We know that the number of valence electron in central metal atom Iodine is seven and in fluorine it is also seven so total number of valence electron in molecule is $7 + 7 \times 7 = 56$.Hence it need seven atomic orbital so possess $s{p^3}{d^3}$ hybridization. The bond angle is ${72^0}$ and ${90^0}$.
Complete step by step solution:
> The given compound is known as iodine heptafluoride. It has seven bond pairs and one lone pair. In the following diagram pentagonal bipyramidal geometry of molecules is shown . > There are two fluorine atoms above and below the plane which make two pyramids with the plane while five fluorine atoms are arranged in a pentagon in the plane. The geometry of molecules used to predict with VSEPR theory (valence shell electron repulsion theory).In this theory the number of electron pairs are used to predict the geometry of molecules.

> In $I{F_7}$ molecule ,central metal atom is $I$. The electronic configuration of iodine atom is given below ;
${I_{53}} = 1{s^2},2{s^2},2{p^6},3{s^2},3{p^6},4{s^2},3{d^{10}},4{p^6},5{s^2},4{d^{10}},5{p^5}$ so here is seven atomic orbital that is one s orbitals, three p orbitals and three d orbitals which hybridizes to form seven hybrid orbitals. The geometry of $I{F_7}$ is pentagonal bipyramidal. Seven hybrid $s{p^3}{d^3}$ orbitals are directed towards the corners of a pentagonal bipyramid. Five of the hybrid orbitals are directed towards the corners of a regular pentagon whereas the remaining two are directed above and below the plane. So here option D is correct.
Note : We know that the number of valence electron in central metal atom Iodine is seven and in fluorine it is also seven so total number of valence electron in molecule is $7 + 7 \times 7 = 56$.Hence it need seven atomic orbital so possess $s{p^3}{d^3}$ hybridization. The bond angle is ${72^0}$ and ${90^0}$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




