
The formula of propane nitrile is:
(A) \[{{C}_{2}}{{H}_{5}}NC\]
(B) \[{{C}_{2}}{{H}_{5}}CN\]
(C) \[C{{H}_{3}}CN\]
(D) \[C{{H}_{3}}NC\]
Answer
241.5k+ views
Hint: We know propane nitrile by the other name of ethyl cyanide and propionitrile. We should know that it is a simple aliphatic nitrile.
Complete step by step answer:
> Propanenitrile is also known by the name of propionitrile, and ethyl cyanide. We should know that it is an organic compound with the formula. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid.
> We can answer the formula of propane nitrile by its name. The name of propane nitrile suggests that it must have three carbon atoms. And the name also suggests that it has a nitrogen atom in it also. So, from the options those are given in this question, we can say that option A and B might be the correct option. Now, we will focus on the last word of propane nitrile that is nitrile. We should know that nitrile is any organic compound that has a −C≡N functional group. Now, we will try to draw the structure from these discussions.
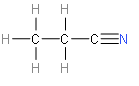
> The above structure represented is the structure of propane nitrile. From the above structure, it is now confirmed that option B is correct. \[{{C}_{2}}{{H}_{5}}CN\] is the correct answer.
Note:It is very important to know about applications of organic compounds such as propane nitrile. We should know that propionitrile is a solvent similar to acetonitrile but with a slightly higher boiling point. We should be careful with propane nitrile because it is poisonous. Propane nitrile has been determined to be teratogenic due to the metabolic release of cyanide. We should know that teratogens are substances that may cause birth defects via a toxic effect on an embryo or foetus.
Complete step by step answer:
> Propanenitrile is also known by the name of propionitrile, and ethyl cyanide. We should know that it is an organic compound with the formula. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid.
> We can answer the formula of propane nitrile by its name. The name of propane nitrile suggests that it must have three carbon atoms. And the name also suggests that it has a nitrogen atom in it also. So, from the options those are given in this question, we can say that option A and B might be the correct option. Now, we will focus on the last word of propane nitrile that is nitrile. We should know that nitrile is any organic compound that has a −C≡N functional group. Now, we will try to draw the structure from these discussions.
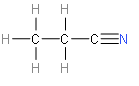
> The above structure represented is the structure of propane nitrile. From the above structure, it is now confirmed that option B is correct. \[{{C}_{2}}{{H}_{5}}CN\] is the correct answer.
Note:It is very important to know about applications of organic compounds such as propane nitrile. We should know that propionitrile is a solvent similar to acetonitrile but with a slightly higher boiling point. We should be careful with propane nitrile because it is poisonous. Propane nitrile has been determined to be teratogenic due to the metabolic release of cyanide. We should know that teratogens are substances that may cause birth defects via a toxic effect on an embryo or foetus.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Inductive Effect and Its Role in Acidic Strength

Understanding Average and RMS Value in Electrical Circuits

JEE Main Correction Window 2026 Session 1 Dates Announced - Edit Form Details, Dates and Link

Other Pages
JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

Iodine is a nonmetal which has metallic luster A True class 11 chemistry JEE_Main

Understanding Electromagnetic Waves and Their Importance

Free Radical Substitution and Its Stepwise Mechanism

How Does Fusion Reaction Happen Inside the Sun?

Understanding the Different Types of Solutions in Chemistry




