
The formal charge on the central oxygen atom in ${{O}_{3}}$ molecule is:
(A) 0
(B) +1
(C) -1
(D) -2
Answer
233.1k+ views
Hint: A formal charge is known to be the no. of valence electrons of an atom minus the no of electrons an atom having within it. The no of valence electrons present in oxygen is 6 and the number of electrons assigned to oxygen is 8.
Complete step by step solution:
From your chemistry lessons you have learned about the formal charge and why it is calculated.
Formal is used to do the comparison between the no. of electrons surrounding the neutral atom versus the no. of electrons surrounding an atom in a molecule.
In molecules, individual atoms are assigned with electron to calculate the formal charge as
-Bonding electrons are divided among the two bonded atoms equally and thus one electron from each bond goes to each atom
-Non-bonding electrons are those electrons which are assigned on those atoms on which they are present.
The formula to calculate the formal charge is,
\[Formal\,charge=(no.\,of\,valence\,electron)-(no.\,of\,non-bonding/lone-pair\,electrons)-\dfrac{1}{2}(no.\,of\,bond\,pair\,electron)\]
Here, we have to calculate the formal charge of the central oxygen atom in ozone $({{O}_{3}})$ molecule,
Firstly we have to draw the lewis dot structure of ozone,
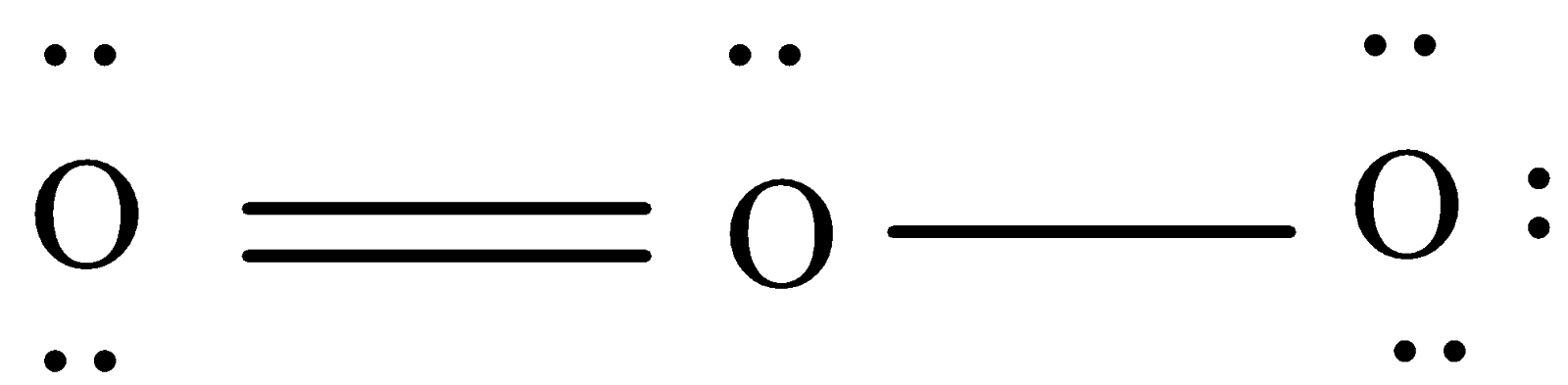
Oxygen has six valence electrons. One oxygen is doubly bonded with the other having 2 lone pairs.
Thus, the total no of valence electrons in an ozone molecule will be 18(3×6).
Now to calculate the formal charge of the central oxygen atom,
the no. of valence electrons= 6
no. of non- bonding or lone pair on central atom =2
no. of bonding electrons of central oxygen atom =6
Bonding electrons will be six because it shares four electrons with the double-bonded oxygen and two electrons with a single bonded oxygen atom.
So, \[Formal\,ch\arg e\,in\,central\,O=(no.\,of\,valence\,electron)-(no.\,of\,non-bonding/lone-pair\,electrons)-\dfrac{1}{2}(no.\,of\,bond\,pair\,electro)\]
\[Formal\,ch\arg e=6-2-\dfrac{1}{2}\times 6=+1\]
Thus the correct option will be (B).
Note: Lone pairs are calculated as non-bonding electrons. Bonding electrons are the shared no. of electrons between the atoms which may be joint through double or single bonds. The sum of formal charge of each atom must be equal to the overall charge of the molecule.
Complete step by step solution:
From your chemistry lessons you have learned about the formal charge and why it is calculated.
Formal is used to do the comparison between the no. of electrons surrounding the neutral atom versus the no. of electrons surrounding an atom in a molecule.
In molecules, individual atoms are assigned with electron to calculate the formal charge as
-Bonding electrons are divided among the two bonded atoms equally and thus one electron from each bond goes to each atom
-Non-bonding electrons are those electrons which are assigned on those atoms on which they are present.
The formula to calculate the formal charge is,
\[Formal\,charge=(no.\,of\,valence\,electron)-(no.\,of\,non-bonding/lone-pair\,electrons)-\dfrac{1}{2}(no.\,of\,bond\,pair\,electron)\]
Here, we have to calculate the formal charge of the central oxygen atom in ozone $({{O}_{3}})$ molecule,
Firstly we have to draw the lewis dot structure of ozone,
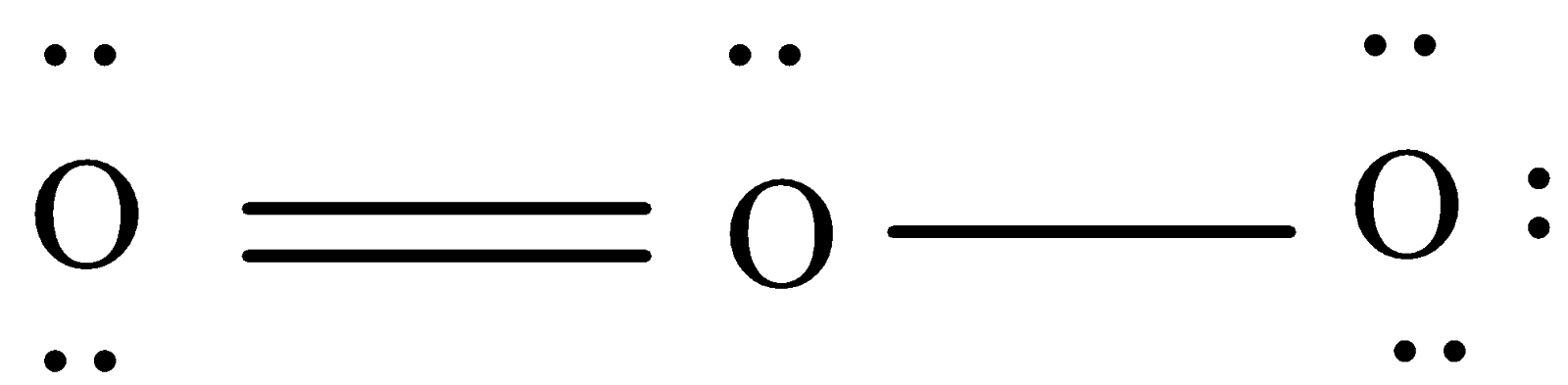
Oxygen has six valence electrons. One oxygen is doubly bonded with the other having 2 lone pairs.
Thus, the total no of valence electrons in an ozone molecule will be 18(3×6).
Now to calculate the formal charge of the central oxygen atom,
the no. of valence electrons= 6
no. of non- bonding or lone pair on central atom =2
no. of bonding electrons of central oxygen atom =6
Bonding electrons will be six because it shares four electrons with the double-bonded oxygen and two electrons with a single bonded oxygen atom.
So, \[Formal\,ch\arg e\,in\,central\,O=(no.\,of\,valence\,electron)-(no.\,of\,non-bonding/lone-pair\,electrons)-\dfrac{1}{2}(no.\,of\,bond\,pair\,electro)\]
\[Formal\,ch\arg e=6-2-\dfrac{1}{2}\times 6=+1\]
Thus the correct option will be (B).
Note: Lone pairs are calculated as non-bonding electrons. Bonding electrons are the shared no. of electrons between the atoms which may be joint through double or single bonds. The sum of formal charge of each atom must be equal to the overall charge of the molecule.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




