
The cyclotron frequency of an electrons gyrating in a magnetic field of $1\,T$ is approximately:
A) $28\,MHz$
B) $280\,MHz$
C) $2.8\,MHz$
D) $28\,GHz$
Answer
241.2k+ views
Hint: To find the value of the frequency of the cyclotron of an electron, use the formula below and substitute the value of the magnetic induction, charge and mass of the electron in it , the obtained result provides the answer for the frequency of cyclotron.
Useful formula:
The formula of the frequency of the cyclotron is given by
$\nu = \dfrac{{qB}}{{2\pi m}}$
Where $\nu $ is the frequency of the cyclotron, $q$ is the charge of the electron, $B$ is the magnetic field and $m$ is the mass.
Complete step by step solution:
It is given that the
Magnetic field that is used in the cyclotron, $B = 1T$
Use the formula of the frequency of the cyclotron,
$\nu = \dfrac{{qB}}{{2\pi m}}$
Substitute the values of the charge $q = 1.6 \times {10^{ - 19}}$ , the magnetic induction from the given, $m = 9.1 \times {10^{ - 31}}$ as the mass of the electron in the above used formula.
$\nu = \dfrac{{1.6 \times {{10}^{ - 19}} \times 1}}{{2 \times 3.14 \times 9.1 \times {{10}^{ - 31}}}}$
Simplifying the above equation, we get
$\nu = \dfrac{{0.8 \times {{10}^{12}}}}{{3.14 \times 9.1}}$
By the further simplification of the above step,
$\nu = 28 \times {10^9}\,Hz$
It is known that the $1\,GHz = {10^9}\,Hz$ , substituting this in the above step
$\nu = 28 \times {10^9}\,GHz$
Hence the cyclotron frequency of the electron is obtained as $\nu = 28\,GHz$. It is not $28\,MHz$ since $1\,MHz = {10^6}\,HZ$ .
Thus the option (D) is correct.
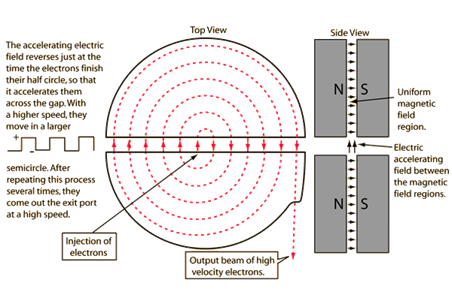
Note: The cyclotron frequency mainly depends upon the specific charge and the magnetic induction. The specific charge is calculated by dividing the charge of the particle and the mass of it. The use of the cyclotron is to produce the radioactive isotopes which are used in the process of the imaging procedures.
Useful formula:
The formula of the frequency of the cyclotron is given by
$\nu = \dfrac{{qB}}{{2\pi m}}$
Where $\nu $ is the frequency of the cyclotron, $q$ is the charge of the electron, $B$ is the magnetic field and $m$ is the mass.
Complete step by step solution:
It is given that the
Magnetic field that is used in the cyclotron, $B = 1T$
Use the formula of the frequency of the cyclotron,

$\nu = \dfrac{{qB}}{{2\pi m}}$
Substitute the values of the charge $q = 1.6 \times {10^{ - 19}}$ , the magnetic induction from the given, $m = 9.1 \times {10^{ - 31}}$ as the mass of the electron in the above used formula.
$\nu = \dfrac{{1.6 \times {{10}^{ - 19}} \times 1}}{{2 \times 3.14 \times 9.1 \times {{10}^{ - 31}}}}$
Simplifying the above equation, we get
$\nu = \dfrac{{0.8 \times {{10}^{12}}}}{{3.14 \times 9.1}}$
By the further simplification of the above step,
$\nu = 28 \times {10^9}\,Hz$
It is known that the $1\,GHz = {10^9}\,Hz$ , substituting this in the above step
$\nu = 28 \times {10^9}\,GHz$
Hence the cyclotron frequency of the electron is obtained as $\nu = 28\,GHz$. It is not $28\,MHz$ since $1\,MHz = {10^6}\,HZ$ .
Thus the option (D) is correct.
Note: The cyclotron frequency mainly depends upon the specific charge and the magnetic induction. The specific charge is calculated by dividing the charge of the particle and the mass of it. The use of the cyclotron is to produce the radioactive isotopes which are used in the process of the imaging procedures.
Recently Updated Pages
JEE Main 2025-26 Mock Tests: Free Practice Papers & Solutions

JEE Main 2025-26 Experimental Skills Mock Test – Free Practice

JEE Main 2025-26 Electronic Devices Mock Test: Free Practice Online

JEE Main 2025-26 Atoms and Nuclei Mock Test – Free Practice Online

JEE Main 2025-26: Magnetic Effects of Current & Magnetism Mock Test

JEE Main Mock Test 2025: Properties of Solids and Liquids

Trending doubts
Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Inductive Effect and Its Role in Acidic Strength

If a wire of resistance R is stretched to double of class 12 physics JEE_Main

Other Pages
JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Class 12 Physics Question Paper Set 3 (55/2/3) 2025: PDF, Answer Key & Solutions

CBSE Class 12 Physics Question Paper Set 3 (55/1/3) 2025 – PDF, Solutions & Analysis

CBSE Class 12 Physics Set 2 (55/2/2) 2025 Question Paper & Solutions

CBSE Class 10 Sanskrit Set 4 52 Question Paper 2025 – PDF, Solutions & Analysis

CBSE Class 12 Physics Question Paper Set 3 (55/4/3) 2025: PDF, Answers & Analysis




