
The correct statement is :
A. most probable velocity of gas molecules increases with the increase in temperature
B. the fraction of gas molecules having most probable speed decreases with the rise in temperature
B. at given temperature, the rms speed of the gas is maximum while most probable speed is maximum
D. All of the above.
Answer
232.8k+ views
Hint : We know that Maxwell’s distribution plot is a plot between the fraction of molecule and speed of molecule at standard temperature. The area under the curve represents the number of molecules. If we heat the gas the peak of the curve will be shifted right because the average speed of the molecule will increase.
Complete step by step solution:
The Maxwell-Boltzmann plot of distribution is given below.
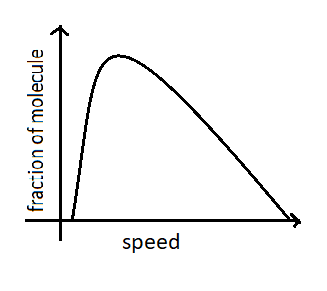
The most probable speed corresponds to the top of the distribution curve because a greater fraction of molecules will have this speed. It is expressed as ${\nu _{mp}} = \sqrt {\dfrac{{2{k_B}T}}{m}} $ where ${k_B}$is Boltzmann constant and $T$ is temperature. We can say that from the equation that the most probable velocity of gas molecules increases with the increase in temperature. At higher temperature, large numbers of molecules will acquire greater amounts of kinetic energy and they move very fast hence their speed increases. The curve also flattens with the increase of temperature. At the same temperature molecules of gas will possess the same kinetic energy. So we can say that when the temperature increases the most probable speed of molecules also increases. Therefore statement A is correct .
Note: We know that we can calculate the most probable speed, the average speed and root mean square speed with the help of Maxwell-Boltzmann equation which gives relation with temperature. Here in the problem we have found out the correct statement with the help of the equation of most probable speed. Second statement is incorrect because it is not possible to decrease the fraction of molecules with the rise of temperature.
Complete step by step solution:
The Maxwell-Boltzmann plot of distribution is given below.

The most probable speed corresponds to the top of the distribution curve because a greater fraction of molecules will have this speed. It is expressed as ${\nu _{mp}} = \sqrt {\dfrac{{2{k_B}T}}{m}} $ where ${k_B}$is Boltzmann constant and $T$ is temperature. We can say that from the equation that the most probable velocity of gas molecules increases with the increase in temperature. At higher temperature, large numbers of molecules will acquire greater amounts of kinetic energy and they move very fast hence their speed increases. The curve also flattens with the increase of temperature. At the same temperature molecules of gas will possess the same kinetic energy. So we can say that when the temperature increases the most probable speed of molecules also increases. Therefore statement A is correct .
Note: We know that we can calculate the most probable speed, the average speed and root mean square speed with the help of Maxwell-Boltzmann equation which gives relation with temperature. Here in the problem we have found out the correct statement with the help of the equation of most probable speed. Second statement is incorrect because it is not possible to decrease the fraction of molecules with the rise of temperature.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




