
The compounds that will give an isomer of 2,2-dimethylpropane on catalytic hydrogenation are
1.
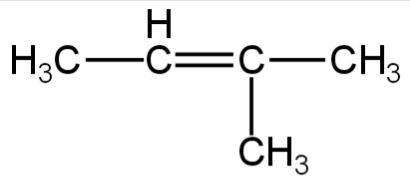
2.
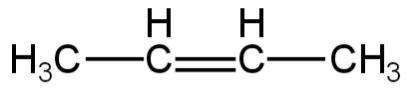
3.
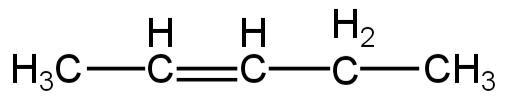
4.
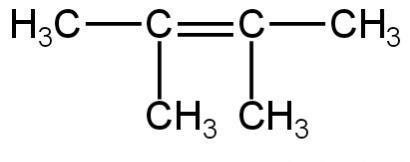
A. 1 and 4
B. 2 and 4
C. 1 and 3
D. 1 and 2
Answer
233.1k+ views
Hint: 2,2-dimethylpropane has five carbon atoms and is also called neopentane. It is a structural isomer of pentane. It is a double-branched-chain alkane. Structural isomers are the compounds having the same molecular formula but different structures
Complete Step by Step Solution:
Here in this question, we are given some hydrocarbons and we have to find out which one of those will give an isomer of 2,2-dimethylpropane on catalytic hydrogenation.
2,2-dimethylpropane as we know is an isomer of the alkane pentane.
So, the hydrocarbon which will have 5 carbon atoms is the isomer of 2,2-dimethylpropane.
1.
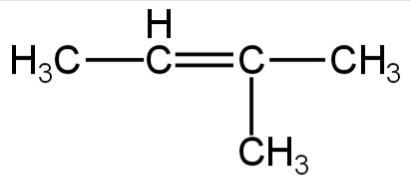
Image: Option 1
This compound on hydrogenation will give 2-methyl butane.
It has five carbon atoms.
So, it is an isomer of 2,2-dimethylpropane.
So, 1 is correct.
2.
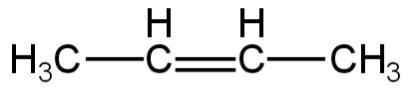
Image: Option 2
This compound on hydrogenation will give butane.
So, it is not an isomer of 2,2-dimethylpropane.
So, 2 is incorrect.
3.
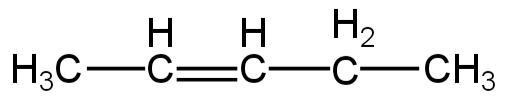
Image: Option 3
This compound on hydrogenation will give pentane.
So, it is an isomer of 2,2-dimethylpropane.
So, 3 is correct.
4.
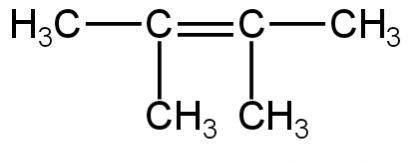
Image: Option 4
This compound on hydrogenation will give 2,3-dimethyl butane. It is an isomer of hexane.
So, 4 is incorrect.
As both options 1 and 3 are correct. So, C is the correct option.
So, option D is correct.
Note: Stereoisomerism is a type of isomerism in which the bonds are similar but the comparative positions of the atoms are different. Enantiomers are a type of stereoisomers that are mirror images of each other that are non-superimposable. These two compounds possess similar physical properties but differ in the path in which they rotate polarised light.
Complete Step by Step Solution:
Here in this question, we are given some hydrocarbons and we have to find out which one of those will give an isomer of 2,2-dimethylpropane on catalytic hydrogenation.
2,2-dimethylpropane as we know is an isomer of the alkane pentane.
So, the hydrocarbon which will have 5 carbon atoms is the isomer of 2,2-dimethylpropane.
1.

Image: Option 1
This compound on hydrogenation will give 2-methyl butane.
It has five carbon atoms.
So, it is an isomer of 2,2-dimethylpropane.
So, 1 is correct.
2.

Image: Option 2
This compound on hydrogenation will give butane.
So, it is not an isomer of 2,2-dimethylpropane.
So, 2 is incorrect.
3.

Image: Option 3
This compound on hydrogenation will give pentane.
So, it is an isomer of 2,2-dimethylpropane.
So, 3 is correct.
4.

Image: Option 4
This compound on hydrogenation will give 2,3-dimethyl butane. It is an isomer of hexane.
So, 4 is incorrect.
As both options 1 and 3 are correct. So, C is the correct option.
So, option D is correct.
Note: Stereoisomerism is a type of isomerism in which the bonds are similar but the comparative positions of the atoms are different. Enantiomers are a type of stereoisomers that are mirror images of each other that are non-superimposable. These two compounds possess similar physical properties but differ in the path in which they rotate polarised light.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




