
The compound 1, 2-butadiene has:
A. Only sp hybridised carbon atoms
B. Only \[s{p^2}\] hybridised carbon atoms
C. Both sp and \[s{p^2}\] hybridised carbon atoms
D. \[sp\], \[s{p^2}\] and \[s{p^3}\] hybridised carbon atoms
Answer
232.8k+ views
Hint: We know that the phenomenon of mixing of orbitals of the same atom with slight difference in energies so as to redistribute the energies and give new orbitals of equivalent energy and shape is termed as hybridization.
Complete step-by-step solution:
Here we first find the hybridisation of each carbon individually either it is \[s{p^2}\], \[s{p^3}\] or \[sp\].
\[s{p^3}\] hybridization uses four \[s{p^3}\] hybridized atomic orbitals. So, there must be the presence of four groups of electrons.
\[s{p^2}\] hybridization uses three \[s{p^2}\] hybridized atomic orbitals. So, there must be the presence of three groups of electrons.
\[sp\] hybridization uses two \[sp\] hybridized atomic orbitals. So, there must be the presence of three groups of electrons.
Let’s come to the question. The structure of 1, 2-butadiene is as follows:
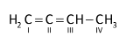
,the 1st carbon is \[s{p^2}\] hybridized as three electrons groups present, 2nd carbon is \[sp\] hybridised (two electron groups) , 3rd carbon is \[s{p^2}\] hybridized (three electrons groups) and 4th carbon is \[s{p^3}\] hybridized because of presence of four electron groups.
Therefore, in 1, 2-butadiene, \[s{p^2}\], \[sp\] and \[s{p^3}\] carbons are present. Hence, D is the correct option.
Note: Hybridisation of carbon atom can be found by counting the number of electron groups surrounding the carbon atom. If four groups present such as in case of \[{\rm{C}}{{\rm{H}}_{\rm{4}}}\] the hybridization is \[s{p^3}\]. If three electron groups are present, hybridization is \[s{p^2}\] and if two electron groups present, hybridization is \[sp\].
Complete step-by-step solution:
Here we first find the hybridisation of each carbon individually either it is \[s{p^2}\], \[s{p^3}\] or \[sp\].
\[s{p^3}\] hybridization uses four \[s{p^3}\] hybridized atomic orbitals. So, there must be the presence of four groups of electrons.
\[s{p^2}\] hybridization uses three \[s{p^2}\] hybridized atomic orbitals. So, there must be the presence of three groups of electrons.
\[sp\] hybridization uses two \[sp\] hybridized atomic orbitals. So, there must be the presence of three groups of electrons.
Let’s come to the question. The structure of 1, 2-butadiene is as follows:
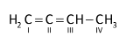
,the 1st carbon is \[s{p^2}\] hybridized as three electrons groups present, 2nd carbon is \[sp\] hybridised (two electron groups) , 3rd carbon is \[s{p^2}\] hybridized (three electrons groups) and 4th carbon is \[s{p^3}\] hybridized because of presence of four electron groups.
Therefore, in 1, 2-butadiene, \[s{p^2}\], \[sp\] and \[s{p^3}\] carbons are present. Hence, D is the correct option.
Note: Hybridisation of carbon atom can be found by counting the number of electron groups surrounding the carbon atom. If four groups present such as in case of \[{\rm{C}}{{\rm{H}}_{\rm{4}}}\] the hybridization is \[s{p^3}\]. If three electron groups are present, hybridization is \[s{p^2}\] and if two electron groups present, hybridization is \[sp\].
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Types of Solutions in Chemistry: Explained Simply

Difference Between Crystalline and Amorphous Solid: Table & Examples

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




