
The C-C bond length of the following molecules is in the order:
\[
{\text{(A)}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ > }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ > }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ > }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}} \\
{\text{(B)}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} \\
{\text{(C) }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} \\
{\text{(D) }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}} \\
\]
Answer
240.9k+ views
Hint: The carbon-carbon bond lengths are dependent upon the type of bonds namely single bond, double bond, or triple bond. The single bond has more bond length compared to double bond which in turn is more than triple bond.
Complete step by step answer:
Bond lengths decrease with increase in s-character. In other words, multiple bonds have a shorter bond length as compared to a single bond.
In the case of single bond only sigma bonds are present whereas in double bond a sigma and a pi bond are present. Sigma bonds are weaker bonds but have high bond length compared to a pi bond. In triple bonds there are two pi bonds which makes it a shorter bond.
A typical carbon-carbon single bond has a length of 154 pm, while a typical double bond and triple bonds are 134 pm and 120 pm, respectively.
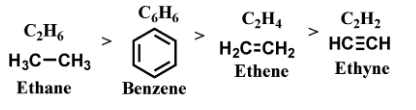
We can see that
Ethane has a single bond between carbon and carbon.
In benzene, the carbon-carbon bond lengths are in resonance due to its aromatic nature, so they have bond length between single bond and double bond as it exhibits partial double bond character.
In ethene, there is a double bond between carbon and carbon.
In ethyne, there is a triple bond between carbon and carbon.
Thus, Single bond > Partial double bond > Double bond > Triple bond.
Therefore, we get the correct following order:
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]
So, the correct option is B.
Note: Partial double bond character is exhibited by molecules having resonance structures where both single bonds and double bonds are exhibited by the molecule. These molecules have bond lengths more than single bonds but less than double bonds.
Complete step by step answer:
Bond lengths decrease with increase in s-character. In other words, multiple bonds have a shorter bond length as compared to a single bond.
In the case of single bond only sigma bonds are present whereas in double bond a sigma and a pi bond are present. Sigma bonds are weaker bonds but have high bond length compared to a pi bond. In triple bonds there are two pi bonds which makes it a shorter bond.
A typical carbon-carbon single bond has a length of 154 pm, while a typical double bond and triple bonds are 134 pm and 120 pm, respectively.

We can see that
Ethane has a single bond between carbon and carbon.
In benzene, the carbon-carbon bond lengths are in resonance due to its aromatic nature, so they have bond length between single bond and double bond as it exhibits partial double bond character.
In ethene, there is a double bond between carbon and carbon.
In ethyne, there is a triple bond between carbon and carbon.
Thus, Single bond > Partial double bond > Double bond > Triple bond.
Therefore, we get the correct following order:
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ < }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{ < }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]
So, the correct option is B.
Note: Partial double bond character is exhibited by molecules having resonance structures where both single bonds and double bonds are exhibited by the molecule. These molecules have bond lengths more than single bonds but less than double bonds.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Chemistry Chapter 9 - Hydrocarbons - 2025-26

CBSE Notes Class 11 Chemistry Chapter 5 - Thermodynamics - 2025-26

CBSE Notes Class 11 Chemistry Chapter 6 - Equilibrium - 2025-26

Inductive Effect and Its Role in Acidic Strength




