
The C — H bond and C— C bond in ethane are formed by which of the following
types of overlap?
(a)- \[s{{p}^{3}}-s\] and \[s{{p}^{3}}-s{{p}^{3}}\]
(b)- \[s{{p}^{2}}-s\] and \[s{{p}^{2}}-s{{p}^{2}}\]
(c)- \[s{{p}^{2}}-s\] and \[sp-sp\]
(d)- \[p-s\]and \[p-p\]
Answer
240.6k+ views
Hint:Covalent bonds are defined as the bonds formed due to sharing of electrons or we can say that they bond through overlapping of atomic orbitals of participant atoms. A covalent bond prefers specific orientations in space.
Complete step by step solution:
> Sigma(σ) bonds can be formed by any one of the following types of combinations of atomic orbitals.
- s-s overlapping: Overlap of two half-filled s-orbitals along the internuclear axis.
- s-p overlapping: Overlap between half-filled s-orbitals of one atom and half-filled p-orbitals of another atom.
- p–p overlapping: Overlap between half-filled p-orbitals of the two approaching atoms.
> First let’s draw the structure of ethane molecules.
> From the structure we can see each carbon forms four bonds, three bonds with a Hydrogen atom and one with the adjacent carbon atom. To find the hybridization of the molecule, first we will isolate both of the Carbon atoms and write down their electron configuration, which is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}\]. From the electronic configuration we can see that only two unpaired electrons are available in orbit but we know that carbon can form four bonds, making it obvious that hybridization is required to make the four unpaired electrons available for this bonding. As a result, four \[s{{p}^{3}}\] hybrid orbitals are formed on each carbon.
> Now, both of the carbons will have four bonds arranged in tetrahedral geometry. The carbon-carbon sigma bond is formed by overlapping of the \[s{{p}^{3}}\] hybrid orbital of the one carbon with the other carbon. which also has three Hydrogens bonded to it in the similar manner., while the six carbon-hydrogen sigma bonds are formed from overlaps between the \[s{{p}^{3}}\] orbitals on the two carbons and the 1s orbitals of six hydrogen
atoms. And we get a molecule with a total of seven sigma bonds and eventually the ethane molecule.
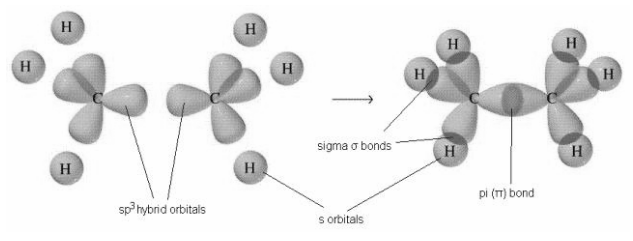
So, the correct option is (a).
Note: C–C bond length is 154 pm and each C–H bond length is 109 pm. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. In this case of ethane molecules, the two methyl (\[C{{H}_{3}}\]) groups are free to rotate.
Complete step by step solution:
> Sigma(σ) bonds can be formed by any one of the following types of combinations of atomic orbitals.
- s-s overlapping: Overlap of two half-filled s-orbitals along the internuclear axis.
- s-p overlapping: Overlap between half-filled s-orbitals of one atom and half-filled p-orbitals of another atom.
- p–p overlapping: Overlap between half-filled p-orbitals of the two approaching atoms.
> First let’s draw the structure of ethane molecules.

> From the structure we can see each carbon forms four bonds, three bonds with a Hydrogen atom and one with the adjacent carbon atom. To find the hybridization of the molecule, first we will isolate both of the Carbon atoms and write down their electron configuration, which is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}\]. From the electronic configuration we can see that only two unpaired electrons are available in orbit but we know that carbon can form four bonds, making it obvious that hybridization is required to make the four unpaired electrons available for this bonding. As a result, four \[s{{p}^{3}}\] hybrid orbitals are formed on each carbon.
> Now, both of the carbons will have four bonds arranged in tetrahedral geometry. The carbon-carbon sigma bond is formed by overlapping of the \[s{{p}^{3}}\] hybrid orbital of the one carbon with the other carbon. which also has three Hydrogens bonded to it in the similar manner., while the six carbon-hydrogen sigma bonds are formed from overlaps between the \[s{{p}^{3}}\] orbitals on the two carbons and the 1s orbitals of six hydrogen
atoms. And we get a molecule with a total of seven sigma bonds and eventually the ethane molecule.

So, the correct option is (a).
Note: C–C bond length is 154 pm and each C–H bond length is 109 pm. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. In this case of ethane molecules, the two methyl (\[C{{H}_{3}}\]) groups are free to rotate.
Recently Updated Pages
Know The Difference Between Fluid And Liquid

Difference Between Crystalline and Amorphous Solid: Table & Examples

Types of Solutions in Chemistry: Explained Simply

Hess Law of Constant Heat Summation: Definition, Formula & Applications

Disproportionation Reaction: Definition, Example & JEE Guide

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

Trending doubts
Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Inductive Effect and Its Role in Acidic Strength

JEE Main Correction Window 2026 Session 1 Dates Announced - Edit Form Details, Dates and Link

Other Pages
JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

Iodine is a nonmetal which has metallic luster A True class 11 chemistry JEE_Main

Understanding Collisions: Types and Examples for Students

Free Radical Substitution and Its Stepwise Mechanism

How Does Fusion Reaction Happen Inside the Sun?

JEE Main 2026 Helpline Numbers for Aspiring Candidates




