
State magnetic properties of ${O_2}$ and ${N_2}.$
Answer
233.1k+ views
Hint: Magnetic property of molecule explains on the basis of molecular orbital theory that, there or two types of molecule i.e., diamagnetic and paramagnetic when atomic orbital contains unpaired electron, it is paramagnetic and if it contains paired electrons the molecule is diamagnetic.
Complete step by step answer:
Let us explain the formation of bonds on the basis of molecular orbital theory.
Nitrogen molecule $[{N_2}]$:
Electronic configuration of N-atom, $1{s^2}2{s^2}2p_x^12p_y^12p_z^1.$
Total number of electrons present in ${N_2}$-molecules is \[14,7\]from each N-atom.
Atomic orbitals of N-atoms form two types of orbitals i.e., bonding and antibonding.
Bonding molecular orbitals form the additive effect of atomic orbitals and antibonding forms subtractive effect of atomic orbital.
Atomic orbitals and relative energies of them are shown in figure.
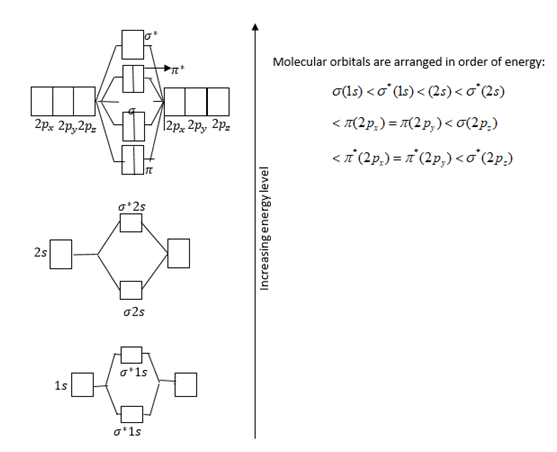
Total electrons filled in orbitals are as follows:
$\sigma {(1s)^2}{\sigma ^*}{(1s)^2}\sigma {(2s)^2}{\sigma ^*}{(2s)^2}\pi {(2{p_x})^2}\pi {(2{p_y})^2}\sigma {(2{p_z})^2}$
Presence of no unpaired electron indicates that ${N_2}$molecule is diamagnetic in nature.
Bond order of ${N_2}$molecule is three, which means that ${N_2}$molecule contains triple bonds.
Bond order$ = \dfrac{{Nb - Na}}{2}$
$ = \dfrac{{8 - 2}}{2}$
Therefore, Bond order $ = 3.$
\[{N_b}\]= Bonding electron
${N_a}$= Antibonding electron
Formation of ${O_2}$ molecule:
Electronic configuration of O-atom is, $1{s^2}2{s^2}2p_x^22p_y^12p_z^1.$
Total number of electrons in the ${O_2}$ molecule is 16. 8 from each O-atom.
14 electrons fill the atomic orbital as has been done in case of ${N_2}$molecule.
The next orbital according to increasing energy ${\pi ^*}(2{p_y}){\pi ^*}(2{p_z})$which have equal energy.
Two more electrons added according to Hund’s Rule.
Therefore, each orbital has one electron.
$\sigma {(1s)^2}{\sigma ^*}{(1s)^2}\sigma {(2s)^2}{\sigma ^*}{(2s)^2}\sigma {(2{p_z})^2}\pi {(2{p_x})^2}\pi {(2{p_y})^2}{\pi ^*}{(2{p_x})^1}{\pi ^*}{(2{p_y})^1}$
Since $\pi $antibonding orbitals have one electron each i.e., unpaired electron.
Therefore, oxygen molecules are paramagnetic in nature.
Therefore, Bond order of ${O_2}$molecule$ = \dfrac{{{N_b} - {N_a}}}{2}$
$ = \dfrac{{8 - 4}}{2}$
Therefore, Bond order $ = 2.$
Therefore, oxygen molecules form double bonds between O-atoms.
Therefore, from the above explanation, the correct option is B
Note: According to molecular orbital theory. Atomic orbitals of combining atoms lose their individual identity. Paramagnetic nature of oxygen and no-existence of $H{e_2},N{e_2}$ molecule explained only by molecular orbital theory.
Complete step by step answer:
Let us explain the formation of bonds on the basis of molecular orbital theory.
Nitrogen molecule $[{N_2}]$:
Electronic configuration of N-atom, $1{s^2}2{s^2}2p_x^12p_y^12p_z^1.$
Total number of electrons present in ${N_2}$-molecules is \[14,7\]from each N-atom.
Atomic orbitals of N-atoms form two types of orbitals i.e., bonding and antibonding.
Bonding molecular orbitals form the additive effect of atomic orbitals and antibonding forms subtractive effect of atomic orbital.
Atomic orbitals and relative energies of them are shown in figure.

Total electrons filled in orbitals are as follows:
$\sigma {(1s)^2}{\sigma ^*}{(1s)^2}\sigma {(2s)^2}{\sigma ^*}{(2s)^2}\pi {(2{p_x})^2}\pi {(2{p_y})^2}\sigma {(2{p_z})^2}$
Presence of no unpaired electron indicates that ${N_2}$molecule is diamagnetic in nature.
Bond order of ${N_2}$molecule is three, which means that ${N_2}$molecule contains triple bonds.
Bond order$ = \dfrac{{Nb - Na}}{2}$
$ = \dfrac{{8 - 2}}{2}$
Therefore, Bond order $ = 3.$
\[{N_b}\]= Bonding electron
${N_a}$= Antibonding electron
Formation of ${O_2}$ molecule:
Electronic configuration of O-atom is, $1{s^2}2{s^2}2p_x^22p_y^12p_z^1.$
Total number of electrons in the ${O_2}$ molecule is 16. 8 from each O-atom.
14 electrons fill the atomic orbital as has been done in case of ${N_2}$molecule.
The next orbital according to increasing energy ${\pi ^*}(2{p_y}){\pi ^*}(2{p_z})$which have equal energy.
Two more electrons added according to Hund’s Rule.
Therefore, each orbital has one electron.
$\sigma {(1s)^2}{\sigma ^*}{(1s)^2}\sigma {(2s)^2}{\sigma ^*}{(2s)^2}\sigma {(2{p_z})^2}\pi {(2{p_x})^2}\pi {(2{p_y})^2}{\pi ^*}{(2{p_x})^1}{\pi ^*}{(2{p_y})^1}$
Since $\pi $antibonding orbitals have one electron each i.e., unpaired electron.
Therefore, oxygen molecules are paramagnetic in nature.
Therefore, Bond order of ${O_2}$molecule$ = \dfrac{{{N_b} - {N_a}}}{2}$
$ = \dfrac{{8 - 4}}{2}$
Therefore, Bond order $ = 2.$
Therefore, oxygen molecules form double bonds between O-atoms.
Therefore, from the above explanation, the correct option is B
Note: According to molecular orbital theory. Atomic orbitals of combining atoms lose their individual identity. Paramagnetic nature of oxygen and no-existence of $H{e_2},N{e_2}$ molecule explained only by molecular orbital theory.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




