
Starting from propanoic acid, the following reactions were carried out
Propanoic acid \[\xrightarrow{{SOC{l_2}}}X\xrightarrow{{N{H_3}}}Y\xrightarrow{{B{r_2} + KOH}}Z\].
What is the compound \[Z\]?
(A) \[C{H_3} - C{H_2} - Br\]
(B) \[C{H_3} - C{H_2} - N{H_2}\]
(C)
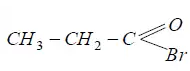
(D) \[C{H_3} - C{H_2} - C{H_2} - N{H_2}\]
Answer
233.1k+ views
Hint: It is possible to classify the propanoic acid formula as a saturated fatty acid. The presence of an ethane molecule connected to the carboxyl group, more precisely to the carbon of the carboxyl group, is the primary property of this short-chain saturated fatty acid. The chemical component, which is often present in a liquid state, has a strong odour attached to it.
Complete Step by Step Solution:
In order to know that propanoic acid has any of the following chemical formulas, even though the first is more frequently used: \[{C_3}{H_6}{O_2}\]or \[C{H_3}C{H_2}COOH\].
Propanoic acid has two different formulae, and its structure can ultimately be illustrated in a variety of ways, including two dimensions and three dimensions, as in the illustrations provided here.
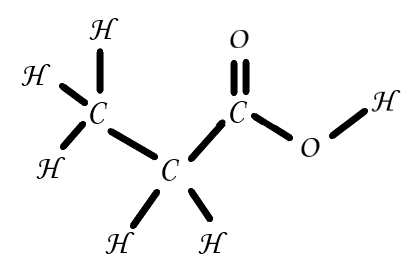
Propanoic acid can be produced in a variety of methods, and is typically thought of as one of the naturally occurring acids. They are created by the Propionibacterium strains of bacteria. This bacterial strain is employed in the biotechnology industry to produce acid at commercial scales. the ability of certain Propionibacterium strains to produce propanoic active. is not frequently utilised for acid bulk synthesis since it is not particularly practical.
Let’ consider the chemical equation of Propanoic acid which is react with \[SOC{l_2}\], then we have:
\[C{H_3}C{H_2}COOH\xrightarrow{{SOC{l_2}}}C{H_3}C{H_2}COCl + S{O_2} + HCl\]
Now, propanoic acid reacts with \[N{H_3}\], then it results:
\[C{H_3}C{H_2}COOH\xrightarrow{{N{H_3}}}C{H_3}C{H_2}CON{H_2} + HCl\]
Similarly, when propanoic acid reacts with \[B{r_2} + KOH\], then we obtain:
\[C{H_3}C{H_2}COOH\xrightarrow{{B{r_2} + KOH}}C{H_3}C{H_2}N{H_2} + C{O_2}\]
Therefore, the compound \[Z\]is \[C{H_3} - C{H_2} - N{H_2}\].
Thus, the correct option is: (B) \[C{H_3} - C{H_2} - N{H_2}\].
Note: It should be noted that propanoic acid is a chemical group with ethane linked to the carboxylic acid group's carbon. The compound's salt and ester are called propionates, and they have a variety of uses. The food business, the pharmaceutical industry, the polymer industry, and the production of animal feed are a few examples of the usage. The chemical and the biological processes both can be used to make propanoic acid. The chemical is produced by the bacteria Propionibacterium in the biotechnology sector.
Complete Step by Step Solution:
In order to know that propanoic acid has any of the following chemical formulas, even though the first is more frequently used: \[{C_3}{H_6}{O_2}\]or \[C{H_3}C{H_2}COOH\].
Propanoic acid has two different formulae, and its structure can ultimately be illustrated in a variety of ways, including two dimensions and three dimensions, as in the illustrations provided here.

Propanoic acid can be produced in a variety of methods, and is typically thought of as one of the naturally occurring acids. They are created by the Propionibacterium strains of bacteria. This bacterial strain is employed in the biotechnology industry to produce acid at commercial scales. the ability of certain Propionibacterium strains to produce propanoic active. is not frequently utilised for acid bulk synthesis since it is not particularly practical.
Let’ consider the chemical equation of Propanoic acid which is react with \[SOC{l_2}\], then we have:
\[C{H_3}C{H_2}COOH\xrightarrow{{SOC{l_2}}}C{H_3}C{H_2}COCl + S{O_2} + HCl\]
Now, propanoic acid reacts with \[N{H_3}\], then it results:
\[C{H_3}C{H_2}COOH\xrightarrow{{N{H_3}}}C{H_3}C{H_2}CON{H_2} + HCl\]
Similarly, when propanoic acid reacts with \[B{r_2} + KOH\], then we obtain:
\[C{H_3}C{H_2}COOH\xrightarrow{{B{r_2} + KOH}}C{H_3}C{H_2}N{H_2} + C{O_2}\]
Therefore, the compound \[Z\]is \[C{H_3} - C{H_2} - N{H_2}\].
Thus, the correct option is: (B) \[C{H_3} - C{H_2} - N{H_2}\].
Note: It should be noted that propanoic acid is a chemical group with ethane linked to the carboxylic acid group's carbon. The compound's salt and ester are called propionates, and they have a variety of uses. The food business, the pharmaceutical industry, the polymer industry, and the production of animal feed are a few examples of the usage. The chemical and the biological processes both can be used to make propanoic acid. The chemical is produced by the bacteria Propionibacterium in the biotechnology sector.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




