
How many S – S bonds, S – O – S bonds, σ-bonds, and π-bonds are present in a trimer of sulphur trioxide?
(A) 0, 3, 16, 2
(B) 0, 3, 12, 6
(C) 0, 6, 12, 16
(D) 0, 4, 12, 6
Answer
233.1k+ views
Hint: Sulphur trioxide forms a cyclic trimer by combining three ${ SO }_{ 3 }$ molecules together. Both the types of bonds can be identified and the number of bonds can be calculated from the structure of the trimer.
Complete answer:
Sulphur trioxide is one of the most well-known chemical compounds that acts as a precursor to the sulfuric acid. Sulphur trioxide has a chemical formula of ${ SO }_{ 3 }$. It can significantly be a primary component of acid rain.
The structure of ${ SO }_{ 3 }$ molecule is given below:
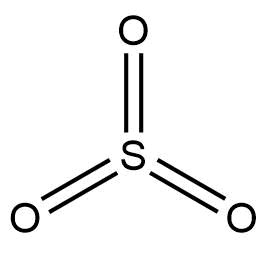
Now, sulphur trioxide can form a cyclic trimer where three ${ SO }_{ 3 }$ molecules are connected together, or it can form a long chain of polymers as well.
In order to determine how many bonds are present and what types of bonds are present in a trimer molecule of sulphur trioxide, then we have to look at its structure. The structure of the trimer of sulphur trioxide is elaborated below:
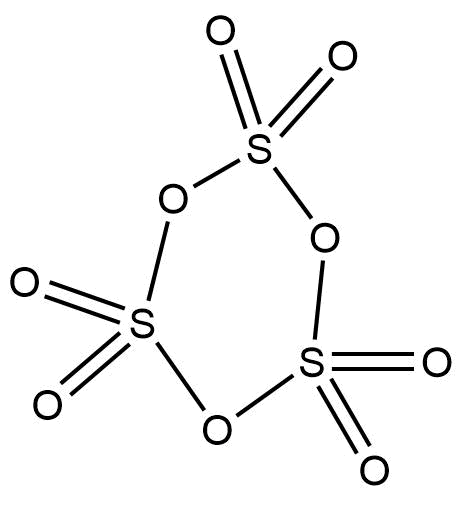
Now, let’s identify how many bonds are present that are mentioned in the question:
* S – S bonds: There is not a single S – S bond is present in the molecule (no two sulphur atoms are directly connected together). Hence, the number is 0.
* S – O – S bonds: There are 3 sulphur atoms in the trimer and each sulphur atom is connected to another sulphur atom via an oxygen atom. Thus, there are three S – O – S bonds. Hence, the number is 3.
* σ-bonds: Any single bond is considered to be a σ-bond. In a double bond, there is one σ-bond and one π-bond. In this molecule, there are 6 σ-bonds counted directly from the single bonds and another 6 σ-bonds that are counted from 6 π-bonds. Thus, there are a total of 12 σ-bonds. Hence, the number is 12.
* π-bonds: It is clearly seen that there are 6 double bonds in this trimer, which means there are 6 π-bonds in this molecule. Hence, the number is 6.
Now, let’s look at the answer options available:
A. 0, 3, 16, 2: The number of σ-bonds and the π-bonds are not 16 and 2 respectively. Hence, option A cannot be correct.
B. 0, 3, 12, 6: All the numbers match with our determination. Hence, option B is the potential answer.
C. 0, 6, 12, 16: The number of S – O – S bonds and π-bonds are not 6 and 16 respectively. Hence, option C cannot be correct.
D. 0, 4, 12, 6: The number of S – O – S bonds is not 4. Hence, option D cannot be correct.
Hence, option B is the correct answer to this question.
Note: To calculate the number of bonds correctly, students have to draw the structure of the trimer correctly. While calculating the σ-bonds, students have to take the double bonds into consideration, because in a double, one is σ-bond and other one is a π-bond.
Complete answer:
Sulphur trioxide is one of the most well-known chemical compounds that acts as a precursor to the sulfuric acid. Sulphur trioxide has a chemical formula of ${ SO }_{ 3 }$. It can significantly be a primary component of acid rain.
The structure of ${ SO }_{ 3 }$ molecule is given below:

Now, sulphur trioxide can form a cyclic trimer where three ${ SO }_{ 3 }$ molecules are connected together, or it can form a long chain of polymers as well.
In order to determine how many bonds are present and what types of bonds are present in a trimer molecule of sulphur trioxide, then we have to look at its structure. The structure of the trimer of sulphur trioxide is elaborated below:

Now, let’s identify how many bonds are present that are mentioned in the question:
* S – S bonds: There is not a single S – S bond is present in the molecule (no two sulphur atoms are directly connected together). Hence, the number is 0.
* S – O – S bonds: There are 3 sulphur atoms in the trimer and each sulphur atom is connected to another sulphur atom via an oxygen atom. Thus, there are three S – O – S bonds. Hence, the number is 3.
* σ-bonds: Any single bond is considered to be a σ-bond. In a double bond, there is one σ-bond and one π-bond. In this molecule, there are 6 σ-bonds counted directly from the single bonds and another 6 σ-bonds that are counted from 6 π-bonds. Thus, there are a total of 12 σ-bonds. Hence, the number is 12.
* π-bonds: It is clearly seen that there are 6 double bonds in this trimer, which means there are 6 π-bonds in this molecule. Hence, the number is 6.
Now, let’s look at the answer options available:
A. 0, 3, 16, 2: The number of σ-bonds and the π-bonds are not 16 and 2 respectively. Hence, option A cannot be correct.
B. 0, 3, 12, 6: All the numbers match with our determination. Hence, option B is the potential answer.
C. 0, 6, 12, 16: The number of S – O – S bonds and π-bonds are not 6 and 16 respectively. Hence, option C cannot be correct.
D. 0, 4, 12, 6: The number of S – O – S bonds is not 4. Hence, option D cannot be correct.
Hence, option B is the correct answer to this question.
Note: To calculate the number of bonds correctly, students have to draw the structure of the trimer correctly. While calculating the σ-bonds, students have to take the double bonds into consideration, because in a double, one is σ-bond and other one is a π-bond.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




