
Predict the product of the following reaction.
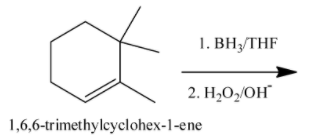
(A) (1R, 2S)-2,3,3-trimethylcyclohexanol + enantiomer
(B) (1R)-1,2,2-trimethylcyclohexanol + enantiomer
(C) (1S,2S)-2,3,3-trimethylcyclohexanol + enantiomer
(D) (1R,2S)-2,3,3-trimethylcyclohexanediol + enantiomer
Answer
233.1k+ views
Hint: The given question is the best example for hydroboration reaction. Alkenes react with hydro boranes and form saturated compounds as products. Highly substituted alkenes also undergo hydroboration and form saturated alkanes.
Complete step by step solution:
-In the question, the name of the reactant is 1,6,6-trimethylcyclohex-1-ene.
-The reaction takes place at an unsaturated centre, meaning a double bond.
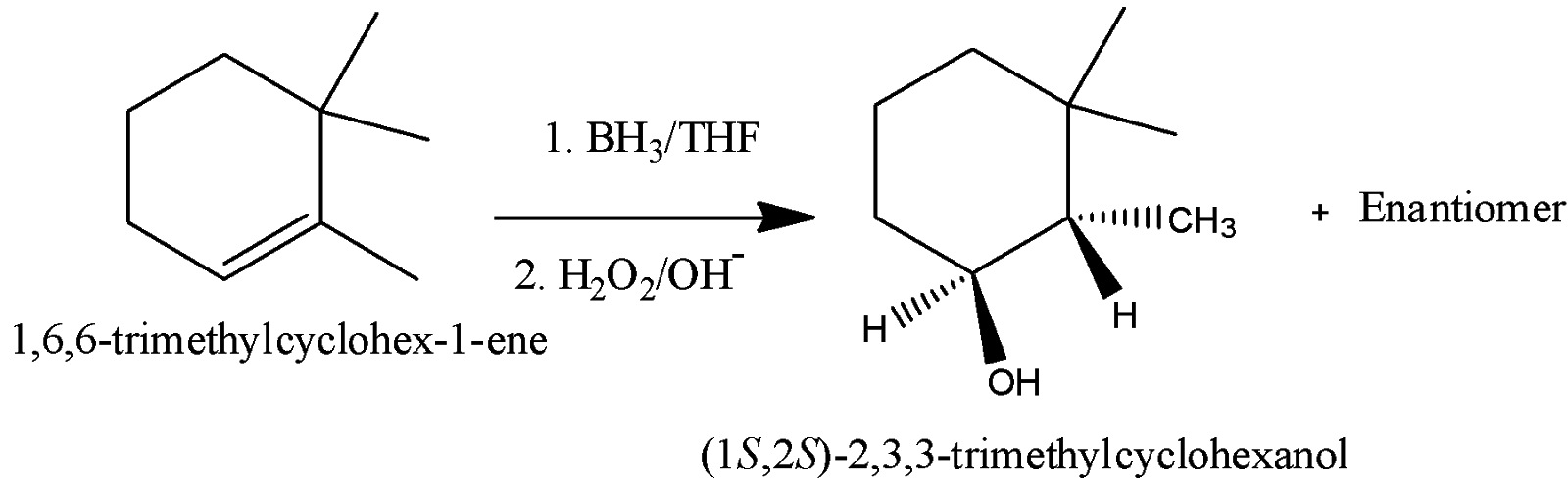
-The complete reaction is as follows.
-Mechanism of hydroboration is as follows.
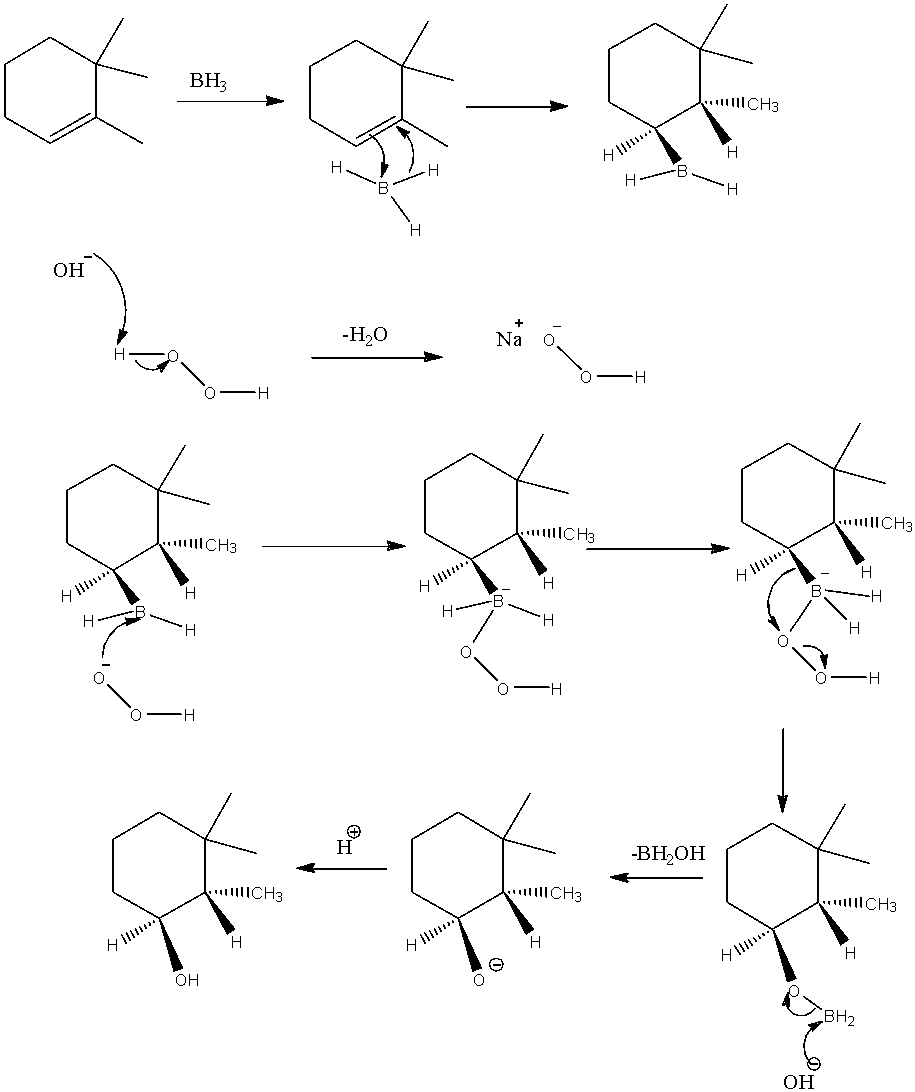
-The advantage with the hydroboration is it undergoes hydrogenation on the same side on the double bond means cis addition happens in presence of hydro boranes.
-The given reaction is called hydroboration-oxidation of Alkenes.
-There is a formation of two products. But the major product is the cis form.
-There is a formation of enantiomers in minor quantities.
-Coming to options, option A, (1R, 2S)-2,3,3-trimethylcyclohexanol + enantiomer, it is wrong because at carbon-1 the configuration is S.
-Coming to option B, (1R)-1,2,2-trimethylcyclohexanol + enantiomer, it is wrong because there are two chiral centres. In the name of the product, only one configuration is mentioned even though the product contains two chiral centres.
-Coming to option D, (1R,2S)-2,3,3-trimethyl cyclohexane diol + enantiomer, it is wrong because at carbon-1 the configuration is S and the name of the product is also wrong.
-The IUPAC name of the formed product is (1S, 2S)-2,3,3-trimethylcyclohexanol.
So, the correct option is (C).
Note: Hydroboration-Oxidation is a two-step pathway used to prepare alcohols from alkenes. This reaction generally uses in the industry to prepare the cis type of alcohols from highly substituted alkenes.
Complete step by step solution:
-In the question, the name of the reactant is 1,6,6-trimethylcyclohex-1-ene.
-The reaction takes place at an unsaturated centre, meaning a double bond.
-The complete reaction is as follows.

-Mechanism of hydroboration is as follows.

-The advantage with the hydroboration is it undergoes hydrogenation on the same side on the double bond means cis addition happens in presence of hydro boranes.
-The given reaction is called hydroboration-oxidation of Alkenes.
-There is a formation of two products. But the major product is the cis form.
-There is a formation of enantiomers in minor quantities.
-Coming to options, option A, (1R, 2S)-2,3,3-trimethylcyclohexanol + enantiomer, it is wrong because at carbon-1 the configuration is S.
-Coming to option B, (1R)-1,2,2-trimethylcyclohexanol + enantiomer, it is wrong because there are two chiral centres. In the name of the product, only one configuration is mentioned even though the product contains two chiral centres.
-Coming to option D, (1R,2S)-2,3,3-trimethyl cyclohexane diol + enantiomer, it is wrong because at carbon-1 the configuration is S and the name of the product is also wrong.
-The IUPAC name of the formed product is (1S, 2S)-2,3,3-trimethylcyclohexanol.
So, the correct option is (C).
Note: Hydroboration-Oxidation is a two-step pathway used to prepare alcohols from alkenes. This reaction generally uses in the industry to prepare the cis type of alcohols from highly substituted alkenes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




