




How Does the Photoelectric Effect Work in Physics?
The photoelectric effect is a fundamental phenomenon in modern physics where electrons are emitted from the surface of a metal when light of sufficient frequency is incident upon it. This effect provided crucial evidence for the quantum nature of light and led to significant developments in quantum mechanics and our understanding of electromagnetic radiation.
Definition and Essential Concept
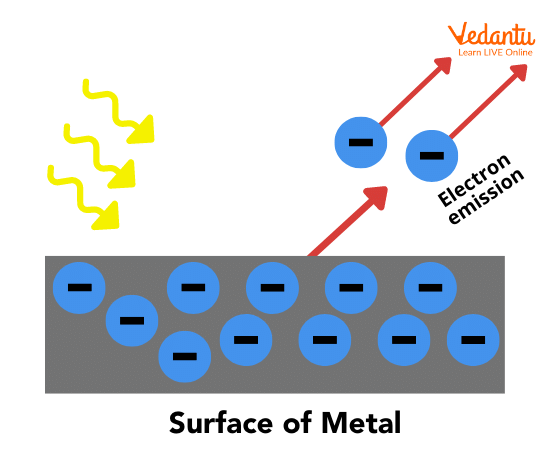
The photoelectric effect refers to the emission of electrons, called photoelectrons, from a metal surface when it is exposed to electromagnetic radiation of energy equal to or greater than a specific minimum value. This minimum energy is known as the work function of the material.
In the classical wave theory of light, it was expected that increasing the intensity of light would release electrons irrespective of frequency, but experimental results contradicted this and necessitated a quantum approach, subsequently explained through the photon theory of light.
Photon Theory and Energy Quantisation
Einstein extended Planck's quantum hypothesis and proposed that light consists of discrete packets of energy called photons. The energy $E$ of a photon is proportional to the frequency $\nu$ of the radiation and is given by:
$E = h\nu = \dfrac{hc}{\lambda}$
Here, $h$ is Planck's constant, $c$ is the speed of light, and $\lambda$ is the wavelength. The photon model accounts for the threshold frequency below which the photoelectric effect does not occur regardless of light intensity.
Types of Electron Emission
Electron emission from the surface of metals can occur by various mechanisms, depending on the form of energy supplied. These types are essential for understanding related phenomena and applications.
- Thermionic emission involves heating the metal
- Field emission occurs by applying a high electric field
- Photoelectric emission is caused by incident light
Photoelectric emission is distinct as it directly demonstrates the particle nature of light and is the primary focus in [Understanding the Photoelectric Effect](https://www.vedantu.com/jee-main/physics-photoelectric-effect).
Photoelectric Effect Equation and Fundamental Formulae
When a photon strikes the metal, its energy can be used to overcome the work function $\phi$ of the material, and any excess energy is imparted as the kinetic energy $K_{\text{max}}$ of the emitted electron. The photoelectric equation is given by the following relation:
$h\nu = \phi + K_{\text{max}}$
The threshold frequency $\nu_{th}$ is the minimum frequency required to release electrons and is related to the work function:
$\phi = h\nu_{th}$
The maximum kinetic energy of the emitted electrons increases linearly with the frequency of incident light but is independent of its intensity.
| Concept/Quantity | Equation |
|---|---|
| Photon Energy | $E = h\nu$ |
| Work Function | $\phi = h\nu_{th}$ |
| Photoelectric Equation | $h\nu = \phi + K_{max}$ |
| Photon Momentum | $E = p c$ |
Internal and External Photoelectric Effects
The photoelectric effect is categorised into two types based on the location of electron emission with respect to the material's surface. These are the internal and external photoelectric effects.
The internal photoelectric effect, also termed the photoconductive or photovoltaic effect, involves electrons gaining sufficient energy to jump from the valence band to the conduction band within the material. This process is the underlying principle of solar cells and some photodetectors.
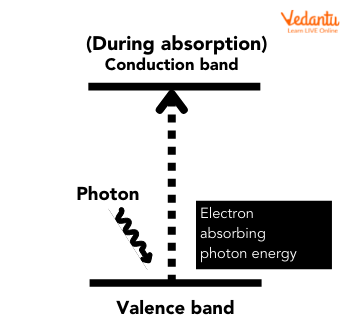
The external photoelectric effect refers to the emission of electrons from the surface of the metal into the surrounding vacuum when photons of energy greater than the work function are incident on the metal surface.
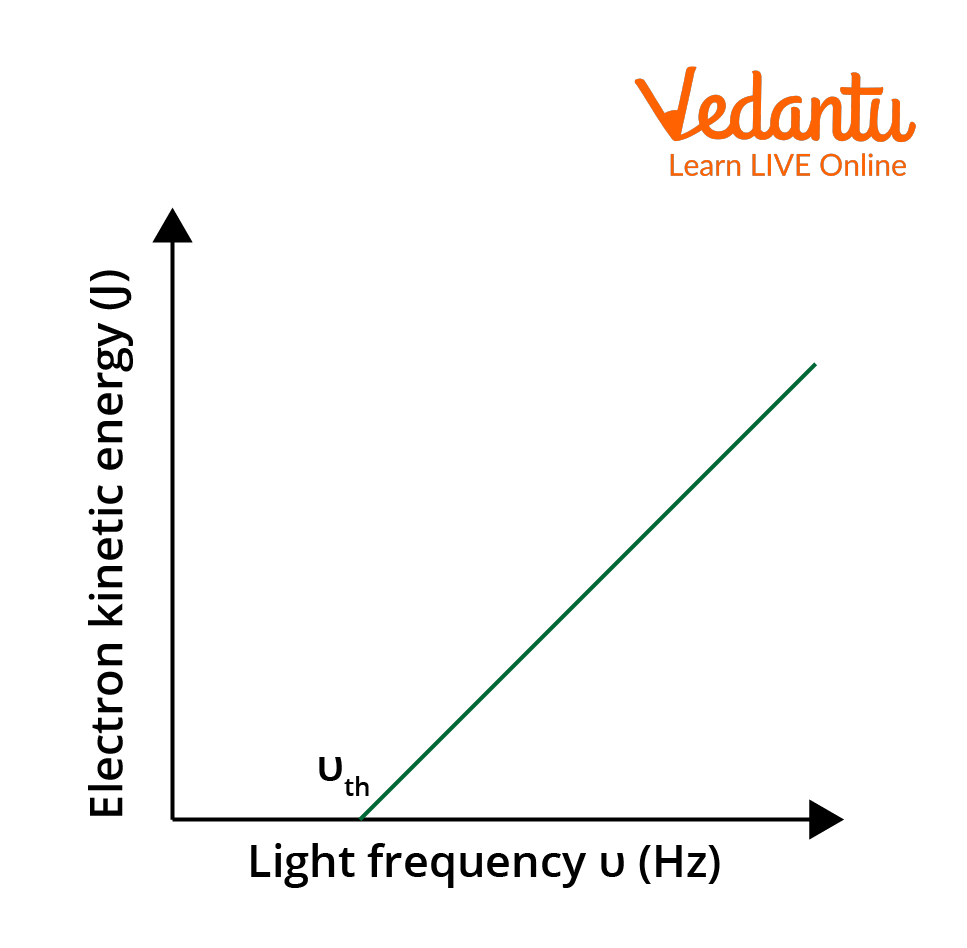
Characteristic Graphs and Experimental Observations
The relationship between the maximum kinetic energy of photoelectrons and the frequency of incident light is linear. The intercept on the frequency axis gives the threshold frequency. The number of emitted photoelectrons increases with intensity but emission only occurs if the frequency exceeds the threshold value.
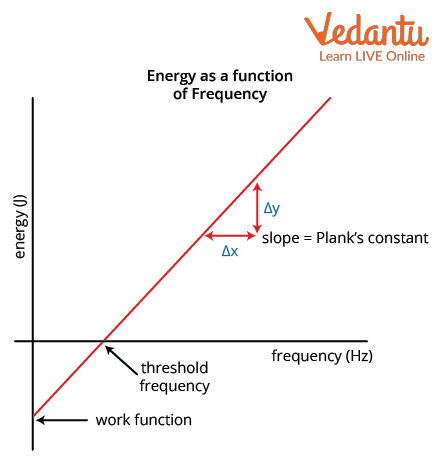
Energy of the emitted photoelectron is expressed as $K_{max} = h\nu - h\nu_{th}$, where $h\nu_{th}$ is the threshold energy required to overcome the work function. Increasing the intensity increases the rate of emission (number of electrons), not their kinetic energy.
Photoelectric Effect Experimental Setup
The classical experiment employs an evacuated glass tube containing a photosensitive emitter and a collector electrode. When monochromatic light of suitable frequency enters, electrons are ejected from the emitter and collected at the cathode, creating a photocurrent. The potential difference is varied to study the behaviour of electron emission with applied voltage.
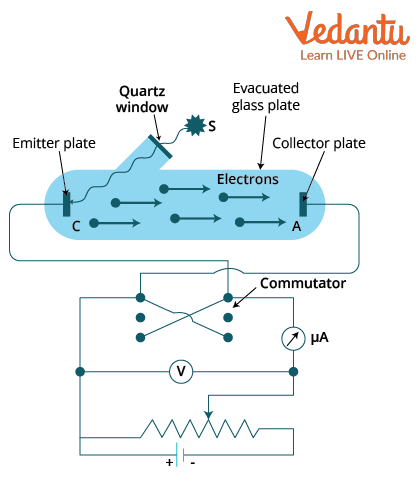
Details on stopping potential and its role in the measurement of photoelectric current are covered in [Photoelectric Effect and Stopping Potential](https://www.vedantu.com/jee-main/physics-photoelectric-effect-and-stopping-potential).
Laws and Properties Related to the Photoelectric Effect
The experimental findings of the photoelectric effect are summarised as follows. There exists a threshold frequency below which no photoemission occurs. Photoelectric current is directly proportional to the intensity of incident radiation, provided frequency exceeds the threshold value. The kinetic energy of emitted electrons depends only on the frequency, not the intensity of the incident light.
- No time lag between illumination and electron emission
- Number of photoelectrons emitted ∝ light intensity
- Kinetic energy of photoelectrons ∝ light frequency
- No electrons emitted below threshold frequency
Properties of Photon
A photon is a quantum of electromagnetic energy with no mass and no electric charge. It travels in vacuum at the speed of light and its energy depends directly on its frequency. Photons exhibit both wave and particle-like properties, pivotal in the quantum explanation of light.
| Property | Description |
|---|---|
| Energy | $E = h\nu$ |
| Momentum | $p = E/c$ |
| Rest mass | $0$ |
| Charge | $0$ |
Applications of the Photoelectric Effect
The photoelectric effect is the foundational principle behind various modern technologies and experimental techniques. The photovoltaic effect is directly utilised in solar panels for electricity generation. Photomultiplier tubes, light sensors, and night vision devices utilise this phenomenon for signal detection and amplification.
Digital cameras and position sensors employ photoelectric sensors for light detection and image formation. X-ray photoelectron spectroscopy (XPS) enables the analysis of elemental composition using the photoelectric effect.
More detailed examples and application cases can be found in [Photoelectric Effect Applications](https://www.vedantu.com/jee-main/physics-photoelectric-effect-and-its-applications).
FAQs on Understanding the Photoelectric Effect: Concepts and Applications
1. What is the photoelectric effect?
The photoelectric effect is the emission of electrons from a metal surface when it is exposed to light of suitable frequency. This phenomenon helped confirm the particle nature of light. Key points include:
- Electrons are released only if the light's frequency is above a certain threshold.
- Emission of electrons is instantaneous after light strikes the surface.
- The energy of emitted electrons depends on the light's frequency, not its intensity.
- This effect supports quantum theory and is key in understanding modern physics and CBSE Class 12 syllabus.
2. Who discovered the photoelectric effect?
Heinrich Hertz first observed the photoelectric effect in 1887, but Albert Einstein explained its theory in 1905, earning the Nobel Prize in Physics in 1921. Major contributors are:
- Heinrich Hertz: Discovered the phenomenon during experiments with radio waves.
- Wilhelm Hallwachs and Philipp Lenard: Conducted related experiments.
- Albert Einstein: Gave the theoretical explanation based on quantum theory and introduced the concept of photons.
3. What is meant by threshold frequency in the photoelectric effect?
Threshold frequency is the minimum frequency of incident light required to emit electrons from a metal surface via the photoelectric effect. Important points:
- Below this frequency, no electrons are emitted regardless of light intensity.
- Threshold frequency depends on the type of metal.
- It connects to the metal's work function.
4. What are the key observations from the photoelectric effect experiment?
The main observations from the photoelectric effect experiment are:
- Electrons are emitted only if the light frequency exceeds a certain threshold.
- Electron emission starts immediately when proper light strikes the surface.
- The number of electrons emitted increases with light intensity (above threshold).
- Kinetic energy of emitted electrons depends on light's frequency, not its intensity.
5. What is Einstein’s photoelectric equation?
Einstein’s photoelectric equation describes the energy balance in the photoelectric effect:
- E = hβ = hβ0 + K.E.
- Here, h is Planck’s constant, β is the frequency of incident light, β0 is the threshold frequency, and K.E. is the maximum kinetic energy of emitted electrons.
- This equation explains why kinetic energy depends on frequency, not intensity, of light.
6. What factors affect the photoelectric current?
The photoelectric current depends on several factors:
- Intensity of incident light: Higher intensity produces more emitted electrons, increasing current.
- Frequency of incident light: Must be above threshold for any current to flow.
- Nature of the metal: Each metal has a different threshold frequency and work function.
- Potential difference: Affects the flow and collection of photoelectrons.
7. What is work function in the context of the photoelectric effect?
Work function is the minimum amount of energy required to remove an electron from a metal surface. Main points:
- It is usually measured in electron volts (eV).
- Work function is specific for each metal.
- Relates to threshold frequency by W = hβ0.
8. Why does increasing intensity of light not increase the kinetic energy of emitted electrons in the photoelectric effect?
Increasing the intensity of light increases the number of emitted electrons, but not their kinetic energy. The kinetic energy of electrons depends only on the frequency of incident light, according to Einstein's photoelectric equation. Thus:
- More intense light → More electrons, same energy (if frequency unchanged).
- Higher frequency light → Greater kinetic energy of each emitted electron.
9. What is the significance of the photoelectric effect in modern physics?
The photoelectric effect is crucial in modern physics because it confirmed the quantum nature of light and supported the idea of photons. Its significance includes:
- Supported Planck’s quantum theory and the existence of discrete energy packets.
- Led to the understanding of the particle-wave duality of light.
- Provided evidence that electromagnetic radiation interacts with matter in discrete energy quanta.
- Applications in photoelectric cells, solar panels, and electronic devices.
10. State the laws of photoelectric emission.
The laws of photoelectric emission are:
- No emission below the threshold frequency, regardless of intensity.
- Emission is instantaneous when suitable light strikes the metal.
- Number of photoelectrons is proportional to the intensity of incident light (above threshold frequency).
- Maximum kinetic energy of photoelectrons depends only on the frequency of the incident light, not its intensity.
























