
Most reactive halide towards substitution nucleophilic unimolecular reaction is
A. n-butyl chloride
B. Sec-butyl chloride
C. Tert-butyl chloride
D. Allyl chloride
Answer
232.8k+ views
Hint: Nucleophilic substitution reactions are the reactions in which an electron-rich nucleophile displaces a functional group forming an electron-deficient molecule or electrophile. Nucleophilic substitution unimolecular has only one species in the rate-determining step.
Complete Step by Step Solution:
An example of a unimolecular substitution reaction is the hydrolysis of 2-bromobutane.
Step-1: Ionisation of 2-bromobutane to form a carbocation intermediate.
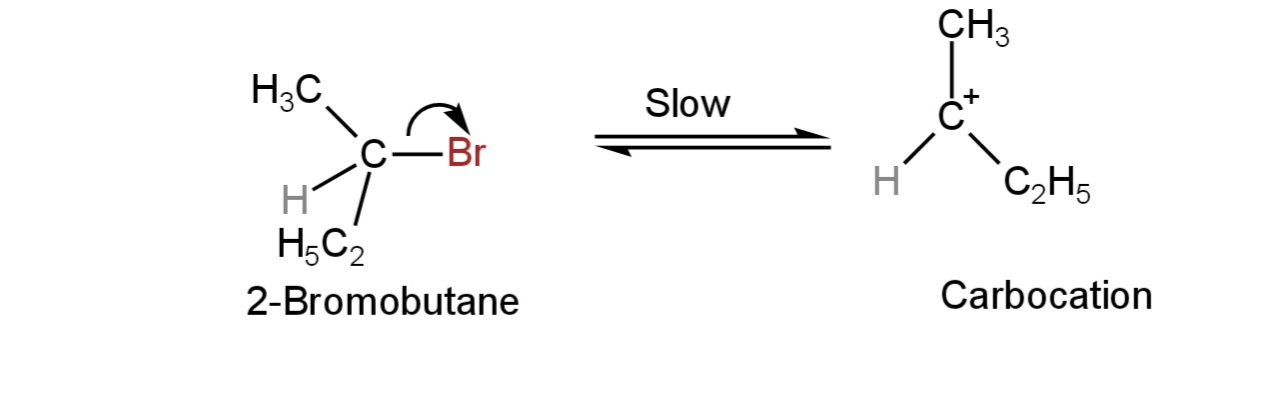
Image: Formation of carbocation
Step 2: Attack by the nucleophile on the carbocation forming the product.
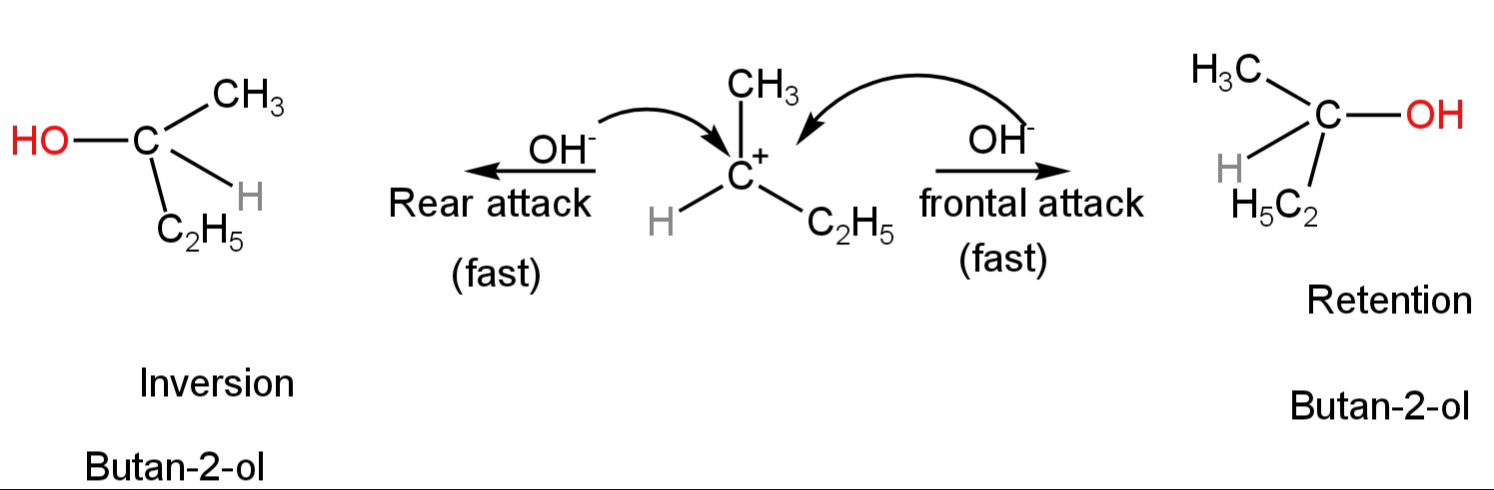
Image: Attack of the nucleophile on the carbocation.
We see that the first step of the reaction is the slow and rate-determining step.
So, the rate of the reaction will depend on the formation of a stable carbocation.
The order of stability of carbocation is Benzyl, alkyl>Tertiary>secondary>primary
A. n-butyl chloride
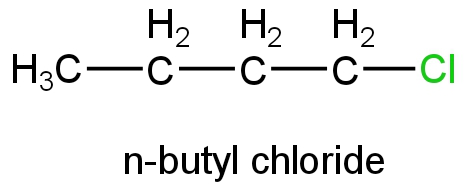
Image: n-butyl chloride
This is a primary halide and its ionisation will form a primary carbocation.
This is unstable due to the negative charge on the carbon atom.
So, it will not react to unimolecular substitution reactions.
B. Sec-butyl chloride
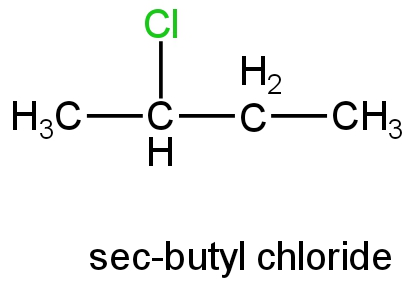
Image: sec-butyl chloride
This is a secondary halide; its ionisation will form a secondary carbocation.
This is more stable than primary halide due to the presence of an electron-donating methyl group which moderately stabilises the negative charge on the carbon atoms. It reacts through a unimolecular substitution reaction.
C. Tert-butyl chloride.
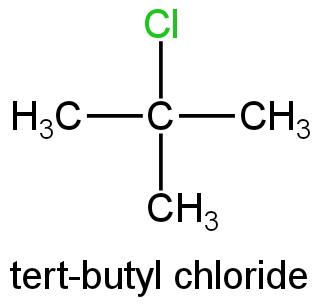
Image: tert-butyl chloride
This is a tertiary halide; its ionisation will form a tert-butyl carbocation.
This carbocation is stable due to the presence of three electron-donating methyl groups which reduces the negative charge of the carbon atom.
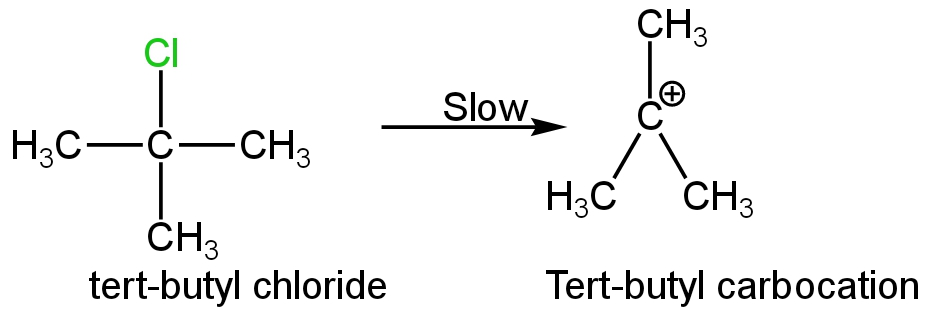
Image: Formation of tert-butyl carbocation
This will react by a unimolecular substitution reaction mechanism.
D. Allyl chloride
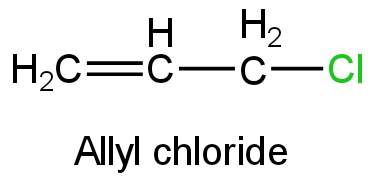
Image: Allyl chloride
Allyl chloride on ionization will form allyl carbocation.
This carbocation is highly stable than secondary and tertiary carbocation due to equivalent resonance.
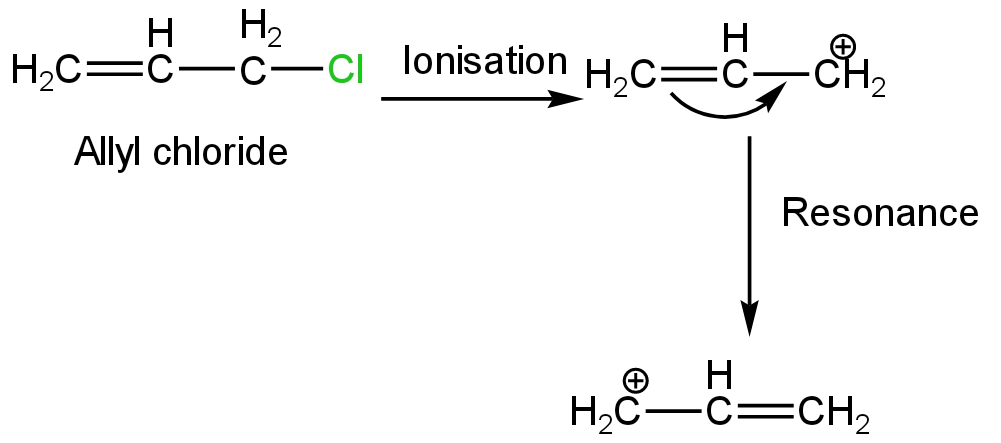
Image: Resonance in allyl carbocation
So, this halide is highly reactive to the unimolecular substitution reaction.
So, option D is correct.
Note: While attending to the question, one has to find the option which will form the most stable carbocation. In this question, sec-butyl chloride and tert-butyl chloride both can react by unimolecular nucleophilic substitution. Out of the given options, allyl chloride due to its most stable carbocation will be most reactive towards unimolecular nucleophilic substitution reaction.
Complete Step by Step Solution:
An example of a unimolecular substitution reaction is the hydrolysis of 2-bromobutane.
Step-1: Ionisation of 2-bromobutane to form a carbocation intermediate.

Image: Formation of carbocation
Step 2: Attack by the nucleophile on the carbocation forming the product.

Image: Attack of the nucleophile on the carbocation.
We see that the first step of the reaction is the slow and rate-determining step.
So, the rate of the reaction will depend on the formation of a stable carbocation.
The order of stability of carbocation is Benzyl, alkyl>Tertiary>secondary>primary
A. n-butyl chloride

Image: n-butyl chloride
This is a primary halide and its ionisation will form a primary carbocation.
This is unstable due to the negative charge on the carbon atom.
So, it will not react to unimolecular substitution reactions.
B. Sec-butyl chloride

Image: sec-butyl chloride
This is a secondary halide; its ionisation will form a secondary carbocation.
This is more stable than primary halide due to the presence of an electron-donating methyl group which moderately stabilises the negative charge on the carbon atoms. It reacts through a unimolecular substitution reaction.
C. Tert-butyl chloride.

Image: tert-butyl chloride
This is a tertiary halide; its ionisation will form a tert-butyl carbocation.
This carbocation is stable due to the presence of three electron-donating methyl groups which reduces the negative charge of the carbon atom.

Image: Formation of tert-butyl carbocation
This will react by a unimolecular substitution reaction mechanism.
D. Allyl chloride

Image: Allyl chloride
Allyl chloride on ionization will form allyl carbocation.
This carbocation is highly stable than secondary and tertiary carbocation due to equivalent resonance.

Image: Resonance in allyl carbocation
So, this halide is highly reactive to the unimolecular substitution reaction.
So, option D is correct.
Note: While attending to the question, one has to find the option which will form the most stable carbocation. In this question, sec-butyl chloride and tert-butyl chloride both can react by unimolecular nucleophilic substitution. Out of the given options, allyl chloride due to its most stable carbocation will be most reactive towards unimolecular nucleophilic substitution reaction.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




