
Molecular shapes of $S{{F}_{4}}$, $C{{F}_{4}}$, $Xe{{F}_{4}}$ are
(A) the same with 2, 0 and 1 lone pairs of electrons respectively
(B) the same with 1,1 and 1 lone pairs of electrons respectively
(C) different with 0, 1 and 2 lone pairs of electrons respectively
(D) different with 1, 0 and 2 lone pairs of electrons respectively
Answer
524.9k+ views
Hint: Valence shell electrons pair theory (VSEPR) suggests that the shape of the molecule depends on the total number of electron pairs in the valence shell of the central atom including bonding and lone pairs. Shapes of the molecules can be derived from hybridization and VSEPR theory.
Complete step by step solution:
Let us try to determine the shapes of the molecules given, i.e. $S{{F}_{4}}$, $C{{F}_{4}}$, $Xe{{F}_{4}}$.
-Shape of $S{{F}_{4}}$molecule
Ground state electronic configuration of S (Z=16): $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3p_{x}^{2}3p_{y}^{1}3p_{z}^{1}$
One electron from the 3d – orbital moves into 3d orbitals so that four electrons can be made available for bonding.
Electronic configuration of S in excited state: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}3{{d}^{1}}$
Five orbitals (one 3s+three 3p+one 3d) hybridize to give $s{{p}^{3}}d$ hybridization which results in the trigonal bipyramidal geometry. Four out of the five $s{{p}^{3}}d$ hybrid orbitals of S are used in bonding with four fluorine atoms whereas one hybrid orbital accommodates the lone pair of electrons.
As per VSEPR theory, S has 6 electrons in the valence shell. In$S{{F}_{4}}$, the total number of electrons on S will be (6+4) = 10. We know that one pair of electrons contains 2 electrons. Therefore, the total number of electron pairs will be 5.
$S{{F}_{4}}$ has 4 bonding pairs of electrons and one lone pair. Lone pair electrons repel the bond pair electrons. Thus, we can have two possible arrangements of the one pair, i.e.
-When the lone pair occupies the axial position.
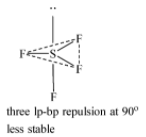
-When the lone pair is occupying one of the three equatorial positions.
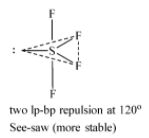
The most stable structure of $S{{F}_{4}}$ is the one where the lone more is present at the equatorial position because there the repulsion is between lone pair and two bond pairs which is less than the repulsion between three lone pairs and bond pair. $S{{F}_{4}}$ has a see-saw type shape.
-Shape of $C{{F}_{4}}$ molecule
Electronic configuration of C (Z = 6) in the ground state: $1{{s}^{2}}2{{s}^{2}}2p_{x}^{1}2p_{y}^{1}$
Electron configuration in the excited state: $1{{s}^{2}}2{{s}^{1}}2p_{x}^{1}2p_{y}^{1}2p_{z}^{1}$
Four orbitals (one 2s +three 2p) undergo hybridization to give four $s{{p}^{3}}$ hybrid orbitals. These orbitals contain four F atoms in the tetrahedral arrangement.
Also, according to VSEPR theory, the four valence electrons on C are shared by four F atoms. These $C-F$ bond pairs repel each other and move apart from one another to attain the shape where the repulsion is the minimum. As a result, the shape obtained is tetrahedral.
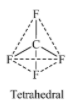
-Shape of $Xe{{F}_{4}}$ molecule
Ground state electronic configuration of Xe (Z = 54): $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$
Outer shell configuration of Xe is $5{{s}^{2}}5{{p}^{6}}$.
There are four bonds present in$Xe{{F}_{4}}$. So, 2 electrons from the 5p – orbitals move into 5d orbitals to make four electrons available for bonding.
Excited state electronic configuration of Xe: $5{{s}^{2}}5{{p}^{4}}5{{d}^{2}}$
Six orbitals (one 5s+three 5p+two 5d) hybridize to give $s{{p}^{3}}{{d}^{2}}$ hybridization. Four out of the six hybrid orbitals contain F atoms while the two orbitals are occupied by lone pairs. $s{{p}^{3}}{{d}^{2}}$ hybridization leads to octahedral geometry but since the two orbitals occupied by lone pairs, the shape of the molecule is square planar.
According to VSEPR theory, $Xe{{F}_{4}}$ has four F atoms. So, the total number of electrons on Xe is (8+4) =12.
Therefore, the total number of electrons pairs available is 6. Out of the six electron pairs, four are bond pairs and two are lone pairs.
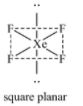
Therefore, we can see that the molecular shape of $S{{F}_{4}}$, $C{{F}_{4}}$ and $Xe{{F}_{4}}$ are different with 1, 0 and 2 lone pairs of electrons.
Hence, the correct option is (D).
Note: Note that if the number of hybrid orbitals is more than the number of atoms or groups surrounding the central atom, i.e. if the total number of electron pairs around the central atom is more than bond pairs, then the shape of the molecule is considered leaving the hybrid orbitals containing lone pairs.
Complete step by step solution:
Let us try to determine the shapes of the molecules given, i.e. $S{{F}_{4}}$, $C{{F}_{4}}$, $Xe{{F}_{4}}$.
-Shape of $S{{F}_{4}}$molecule
Ground state electronic configuration of S (Z=16): $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3p_{x}^{2}3p_{y}^{1}3p_{z}^{1}$
One electron from the 3d – orbital moves into 3d orbitals so that four electrons can be made available for bonding.
Electronic configuration of S in excited state: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}3{{d}^{1}}$
Five orbitals (one 3s+three 3p+one 3d) hybridize to give $s{{p}^{3}}d$ hybridization which results in the trigonal bipyramidal geometry. Four out of the five $s{{p}^{3}}d$ hybrid orbitals of S are used in bonding with four fluorine atoms whereas one hybrid orbital accommodates the lone pair of electrons.
As per VSEPR theory, S has 6 electrons in the valence shell. In$S{{F}_{4}}$, the total number of electrons on S will be (6+4) = 10. We know that one pair of electrons contains 2 electrons. Therefore, the total number of electron pairs will be 5.
$S{{F}_{4}}$ has 4 bonding pairs of electrons and one lone pair. Lone pair electrons repel the bond pair electrons. Thus, we can have two possible arrangements of the one pair, i.e.
-When the lone pair occupies the axial position.

-When the lone pair is occupying one of the three equatorial positions.

The most stable structure of $S{{F}_{4}}$ is the one where the lone more is present at the equatorial position because there the repulsion is between lone pair and two bond pairs which is less than the repulsion between three lone pairs and bond pair. $S{{F}_{4}}$ has a see-saw type shape.
-Shape of $C{{F}_{4}}$ molecule
Electronic configuration of C (Z = 6) in the ground state: $1{{s}^{2}}2{{s}^{2}}2p_{x}^{1}2p_{y}^{1}$
Electron configuration in the excited state: $1{{s}^{2}}2{{s}^{1}}2p_{x}^{1}2p_{y}^{1}2p_{z}^{1}$
Four orbitals (one 2s +three 2p) undergo hybridization to give four $s{{p}^{3}}$ hybrid orbitals. These orbitals contain four F atoms in the tetrahedral arrangement.
Also, according to VSEPR theory, the four valence electrons on C are shared by four F atoms. These $C-F$ bond pairs repel each other and move apart from one another to attain the shape where the repulsion is the minimum. As a result, the shape obtained is tetrahedral.

-Shape of $Xe{{F}_{4}}$ molecule
Ground state electronic configuration of Xe (Z = 54): $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$
Outer shell configuration of Xe is $5{{s}^{2}}5{{p}^{6}}$.
There are four bonds present in$Xe{{F}_{4}}$. So, 2 electrons from the 5p – orbitals move into 5d orbitals to make four electrons available for bonding.
Excited state electronic configuration of Xe: $5{{s}^{2}}5{{p}^{4}}5{{d}^{2}}$
Six orbitals (one 5s+three 5p+two 5d) hybridize to give $s{{p}^{3}}{{d}^{2}}$ hybridization. Four out of the six hybrid orbitals contain F atoms while the two orbitals are occupied by lone pairs. $s{{p}^{3}}{{d}^{2}}$ hybridization leads to octahedral geometry but since the two orbitals occupied by lone pairs, the shape of the molecule is square planar.
According to VSEPR theory, $Xe{{F}_{4}}$ has four F atoms. So, the total number of electrons on Xe is (8+4) =12.
Therefore, the total number of electrons pairs available is 6. Out of the six electron pairs, four are bond pairs and two are lone pairs.

Therefore, we can see that the molecular shape of $S{{F}_{4}}$, $C{{F}_{4}}$ and $Xe{{F}_{4}}$ are different with 1, 0 and 2 lone pairs of electrons.
Hence, the correct option is (D).
Note: Note that if the number of hybrid orbitals is more than the number of atoms or groups surrounding the central atom, i.e. if the total number of electron pairs around the central atom is more than bond pairs, then the shape of the molecule is considered leaving the hybrid orbitals containing lone pairs.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




