
Main Products formed during a reaction of 1-methoxy naphthalene with hydroiodic acid are:
A.
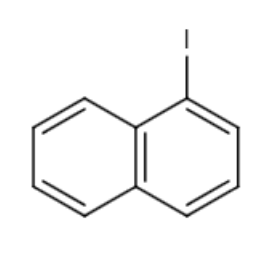 and CH3I
and CH3I
B.
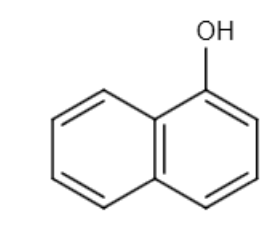 and CH3OH
and CH3OH
C.
 and CH3OH
and CH3OH
D.
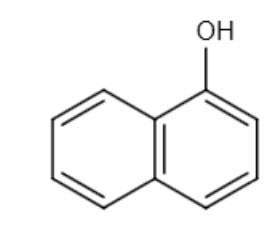 and CH3I
and CH3I
Answer
233.1k+ views
Hint: Here, in this question, 1-methoxy naphthalene is given. It reacts with hydroiodic acid. First of all, hydrogen ions react with 1-methoxy naphthalene followed by iodide ions.
Complete Step by Step Solution:
The structure of 1-methoxy naphthalene is as follows:
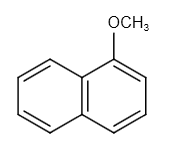
1-methoxy naphthalene reacts with hydrogen ion as follows:
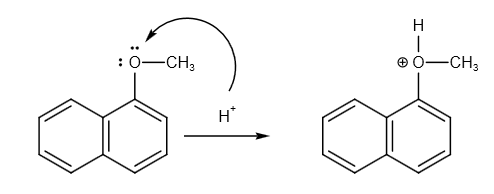
Hydrogen ions attack oxygen and bind with oxygen.
It will further react with iodine ion as follows:
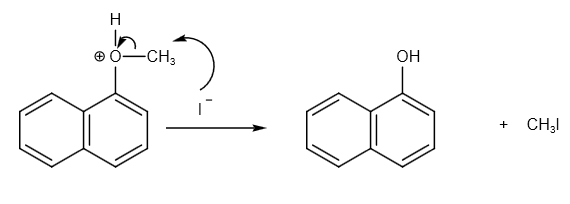
The carbon and oxygen bond breaks and iodine ions attack carbon to form methyl iodide.
Therefore, the correct answer is option D.
Additional Information:
The odour of naphthalene, a white, crystalline volatile substance, is often compared to that of mothballs. The substance slowly sublimes (changes from a solid to a gas) at room temperature, producing a vapour that is extremely combustible. The structure of naphthalene can be used to predict how soluble it is in water. A polyatomic hydrocarbon is naphthene. It is a hydrophobic molecule since it contains a lot of carbon atoms. The hydrophobic nature of this naphthalene makes it insoluble in water. The liquid nature of naphthalene is not thoroughly covered in this article. In 1-methoxy naphthalene, there is one hydrogen of naphthalene substituted by the methyl group.
Note: We must keep in mind that hydrogen iodide is a halide of hydrogen and a diatomic molecule. Aqueous solutions of HI are also referred to as hydroiodic acid, a solid acid. Hydroiodic acid and hydrogen iodide, however, are different in that the former is a gas under normal circumstances while the latter is an aqueous gas solution.
Complete Step by Step Solution:
The structure of 1-methoxy naphthalene is as follows:

1-methoxy naphthalene reacts with hydrogen ion as follows:

Hydrogen ions attack oxygen and bind with oxygen.
It will further react with iodine ion as follows:

The carbon and oxygen bond breaks and iodine ions attack carbon to form methyl iodide.
Therefore, the correct answer is option D.
Additional Information:
The odour of naphthalene, a white, crystalline volatile substance, is often compared to that of mothballs. The substance slowly sublimes (changes from a solid to a gas) at room temperature, producing a vapour that is extremely combustible. The structure of naphthalene can be used to predict how soluble it is in water. A polyatomic hydrocarbon is naphthene. It is a hydrophobic molecule since it contains a lot of carbon atoms. The hydrophobic nature of this naphthalene makes it insoluble in water. The liquid nature of naphthalene is not thoroughly covered in this article. In 1-methoxy naphthalene, there is one hydrogen of naphthalene substituted by the methyl group.
Note: We must keep in mind that hydrogen iodide is a halide of hydrogen and a diatomic molecule. Aqueous solutions of HI are also referred to as hydroiodic acid, a solid acid. Hydroiodic acid and hydrogen iodide, however, are different in that the former is a gas under normal circumstances while the latter is an aqueous gas solution.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




