
In\[\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}\], Cu has the following hybridization
A.\[ds{p^2}\]
B.\[s{p^3}\]
C.\[s{p^2}\;\]
D.\[s{p^3}{d^2}\]
Answer
232.8k+ views
Hint: \[\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}\] is a coordination compound.
The shape of coordination compounds is provided by valence bond theory which states that the metal-ligand bond is formed by the donating of pairs of electrons by the ligand to the metal or ion.
Complete step by step solution:We are given a compound \[\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}\]where Cu is the central metal and ammonia is the ligand.
Four molecules of ammonia are bonded to the Cu metal constituting a coordination entity.
The sulfate ion present outside the coordination entity is not bonded to the metal.
The charge on the coordination entity is +2 as the charge of sulfate ion present outside the coordination entity is -2 and the coordination compound is electrically neutral.
NH3 is a neutral ligand.
Let the charge of Cu is x.
So,\[x + 0 = + 2\]
\[ \Rightarrow x = + 2\]
The shape of this compound is predicted by valence bond theory.
Valence bond theory states that the metal-ligand bond is formed by the donation of pairs of electrons by the ligand to the metal or ion.
These electrons are accommodated by the hybridized orbitals of equal energy of metal atoms.
Here in this question, we have to find out the hybridization of Cu.
The electronic configuration of Cu is\[\left[ {Ar} \right]{\rm{ }}3{d^{10}}4{s^1}\].
It has a +2 charge in the given compound.
The new electronic configuration is\[3{d^9}\].
So, the total no.of electrons on the central metal atom=9 i.e.,
These electrons lie in the 3d orbital of the central metal ion with the last electron unpaired.
Ammonia is a strong field ligand. So, it is, therefore, will be able to put the last electron to the last 4p orbital.
The remaining d orbital, one 4s, and two 4p orbitals hybridize to form four hybrid orbitals.
These hybrid orbitals will accommodate four pairs of electrons from ammonia.
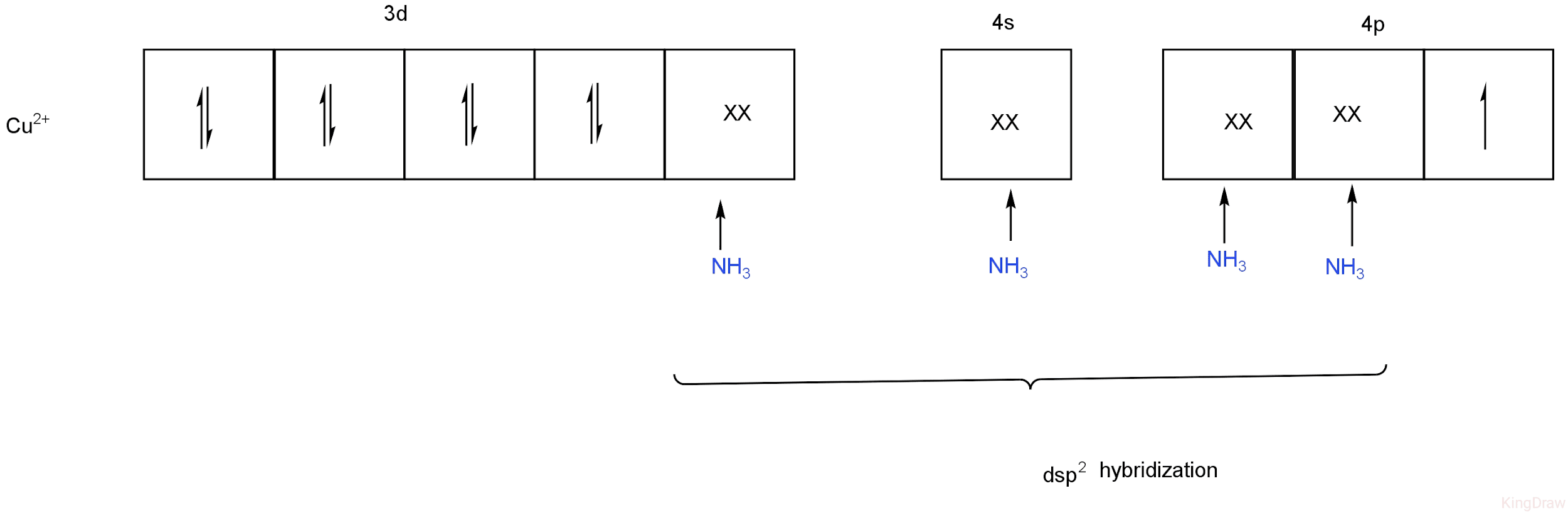
Image:\[ds{p^2}\] hybridization of Cu.
So, in \[\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}\], Cu has the\[ds{p^2}\]hybridization.
So, option A is correct.
Note: It must be noted that even though Cu has a +2 charge and \[3{d^9}\]configuration, it doesn't have the usual sp3 hybridization.
It should not be confused with\[s{p^3}\]as ammonia is a strong ligand it will push the unpaired electron to the 4p orbital.
If there was a weak field ligand, the usual hybridization would have happened.
The shape of coordination compounds is provided by valence bond theory which states that the metal-ligand bond is formed by the donating of pairs of electrons by the ligand to the metal or ion.
Complete step by step solution:We are given a compound \[\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}\]where Cu is the central metal and ammonia is the ligand.
Four molecules of ammonia are bonded to the Cu metal constituting a coordination entity.
The sulfate ion present outside the coordination entity is not bonded to the metal.
The charge on the coordination entity is +2 as the charge of sulfate ion present outside the coordination entity is -2 and the coordination compound is electrically neutral.
NH3 is a neutral ligand.
Let the charge of Cu is x.
So,\[x + 0 = + 2\]
\[ \Rightarrow x = + 2\]
The shape of this compound is predicted by valence bond theory.
Valence bond theory states that the metal-ligand bond is formed by the donation of pairs of electrons by the ligand to the metal or ion.
These electrons are accommodated by the hybridized orbitals of equal energy of metal atoms.
Here in this question, we have to find out the hybridization of Cu.
The electronic configuration of Cu is\[\left[ {Ar} \right]{\rm{ }}3{d^{10}}4{s^1}\].
It has a +2 charge in the given compound.
The new electronic configuration is\[3{d^9}\].
So, the total no.of electrons on the central metal atom=9 i.e.,
These electrons lie in the 3d orbital of the central metal ion with the last electron unpaired.
Ammonia is a strong field ligand. So, it is, therefore, will be able to put the last electron to the last 4p orbital.
The remaining d orbital, one 4s, and two 4p orbitals hybridize to form four hybrid orbitals.
These hybrid orbitals will accommodate four pairs of electrons from ammonia.

Image:\[ds{p^2}\] hybridization of Cu.
So, in \[\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]S{O_4}\], Cu has the\[ds{p^2}\]hybridization.
So, option A is correct.
Note: It must be noted that even though Cu has a +2 charge and \[3{d^9}\]configuration, it doesn't have the usual sp3 hybridization.
It should not be confused with\[s{p^3}\]as ammonia is a strong ligand it will push the unpaired electron to the 4p orbital.
If there was a weak field ligand, the usual hybridization would have happened.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




