
In the Boyle's law experiment, the graph drawn between pressure and density for a given temperature of different gases are the straight lines then the molecular weights have the relation
A) ${M_1} > {M_2}$
B) ${M_2} > {M_1}$
C) ${M_1} = {M_2}$
D) $M_2^2 = {M_1}$
Answer
240.3k+ views
Hint: The graph is between pressure and density. To find the relation between the two masses can be obtained by using the expression for density and Boyle's law for an ideal gas. The density of a gas is the ratio of its mass to the volume. Boyle's law graphs are plotted with pressure on one axis and reciprocal of volume on the other axis. In this graph mass of the gas is multiplied by the volume to get density on the x-axis.
Formula used:
$d = \dfrac{m}{V}$ (Where d stands for the density, m stands for the given mass and V stands for the volume)
The ideal gas equation is given by,
$PV = nRT$ (where P is the pressure of the gas, V is the volume of the gas, n stands for the number of moles, R stands for the universal gas constant and T is the temperature of the gas)
Complete step by step solution:
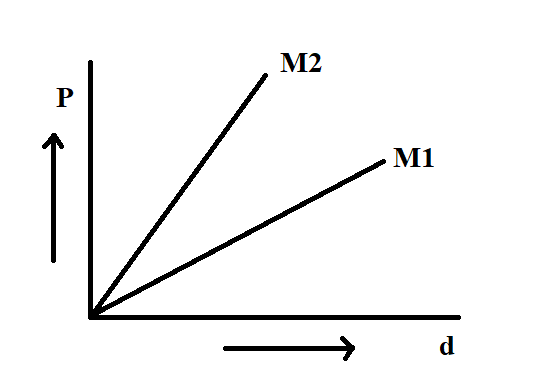
We know that in the graph, pressure versus density is shown,
We know the expression for density,
Density $d = $$\dfrac{{mass}}{{volume}} = \dfrac{m}{V}$
Boyle's law for ideal gases is given by,
$PV = nRT$
$n$ is the number of moles which is given by, $\dfrac{{mass(given)}}{{Mass(molecular)}} = \dfrac{m}{M}$
where$ M$ is the molecular weight.
Substituting the value of $n$ in the ideal gas equation,
$PV = \dfrac{m}{M}RT$
Rearranging this equation, we get the density
Density, $(d)= \dfrac{m}{V} = \dfrac{{PM}}{{RT}}$
The graph of pressure versus density is shown in the figure as a straight line.
From the equation, we get that the slope of this line is, $\dfrac{{RT}}{M}$
As the molecular mass is in the denominator, the line with a greater slope will be having less molecular mass.
Since ${M_2}$ is steeper than ${M_1}$, ${M_1}$ will be greater than ${M_2}$.
Hence, the answer is (A), ${M_1} > {M_2}$.
Note: The slope will be more for a steeper line. Boyle’s law states that for a constant temperature the volume of a given mass of a gas is inversely proportional to volume. Gases are liquefied by using the principle of Boyle’s law. Boyle's law has many real-life applications also. The injection syringe is one example of Boyle's law.
Formula used:
$d = \dfrac{m}{V}$ (Where d stands for the density, m stands for the given mass and V stands for the volume)
The ideal gas equation is given by,
$PV = nRT$ (where P is the pressure of the gas, V is the volume of the gas, n stands for the number of moles, R stands for the universal gas constant and T is the temperature of the gas)
Complete step by step solution:
We know that in the graph, pressure versus density is shown,
We know the expression for density,
Density $d = $$\dfrac{{mass}}{{volume}} = \dfrac{m}{V}$
Boyle's law for ideal gases is given by,
$PV = nRT$
$n$ is the number of moles which is given by, $\dfrac{{mass(given)}}{{Mass(molecular)}} = \dfrac{m}{M}$
where$ M$ is the molecular weight.
Substituting the value of $n$ in the ideal gas equation,
$PV = \dfrac{m}{M}RT$
Rearranging this equation, we get the density
Density, $(d)= \dfrac{m}{V} = \dfrac{{PM}}{{RT}}$
The graph of pressure versus density is shown in the figure as a straight line.
From the equation, we get that the slope of this line is, $\dfrac{{RT}}{M}$
As the molecular mass is in the denominator, the line with a greater slope will be having less molecular mass.
Since ${M_2}$ is steeper than ${M_1}$, ${M_1}$ will be greater than ${M_2}$.
Hence, the answer is (A), ${M_1} > {M_2}$.
Note: The slope will be more for a steeper line. Boyle’s law states that for a constant temperature the volume of a given mass of a gas is inversely proportional to volume. Gases are liquefied by using the principle of Boyle’s law. Boyle's law has many real-life applications also. The injection syringe is one example of Boyle's law.
Recently Updated Pages
Dimensions of Charge: Dimensional Formula, Derivation, SI Units & Examples

How to Calculate Moment of Inertia: Step-by-Step Guide & Formulas

Circuit Switching vs Packet Switching: Key Differences Explained

Dimensions of Pressure in Physics: Formula, Derivation & SI Unit

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE General Topics in Chemistry Important Concepts and Tips

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Clemmensen and Wolff Kishner Reductions Explained for JEE & NEET

Degree of Dissociation: Meaning, Formula, Calculation & Uses

Understanding the Angle of Deviation in a Prism

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

CBSE Notes Class 11 Physics Chapter 4 - Laws of Motion - 2025-26

CBSE Notes Class 11 Physics Chapter 14 - Waves - 2025-26

CBSE Notes Class 11 Physics Chapter 9 - Mechanical Properties of Fluids - 2025-26

Inductive Effect and Its Role in Acidic Strength




