
In ${{\left( C{{H}_{3}} \right)}_{3}}N$ nitrogen is $s{{p}^{3}}$ hybridised where as in ${{\left( Si{{H}_{3}} \right)}_{3}}N$ it is $s{{p}^{2}}$ hybridised, why?
Answer
233.1k+ views
Hint: To solve this approach through the bonding concept. One here will undergo back-bonding which will force it to take up a planar structure. The one which is the bulkier group here will take up the geometry with wider bond-angle due to steric repulsion.
Complete step by step solution:
We know that according to the VSEPR theory that is the Valence Shell Electron Pair Repulsion theory, the hybridisation of the atom can tell us about its bonding and its structure.
According to VSEPR theory, $s{{p}^{3}}$ hybridisation has a tetrahedral geometry and $s{{p}^{2}}$ hybridisation has a trigonal planar structure. Trigonal planar geometry is planar and tetrahedral geometry is not planar as one of the atoms lie above the plane.
Now in the given question, if we discuss why they take up the particular geometry, we can explain the hybridisation on its basis.
Firstly let’s discuss ${{\left( Si{{H}_{3}} \right)}_{3}}N$. We know that nitrogen has 5 electrons in the valence shell and after forming ${{\left( Si{{H}_{3}} \right)}_{3}}N$, it still has a pair of lone-pair on it. Now, these lone pairs of electrons can undergo back-bonding with the Si-atom as it has energetically accessible d-orbitals which can undergo back-bonding with the lone pair, p-orbital of nitrogen. Due to this $p\pi \left( N \right)\to d\pi \left( Si \right)$ back-bonding it attains a planar structure.
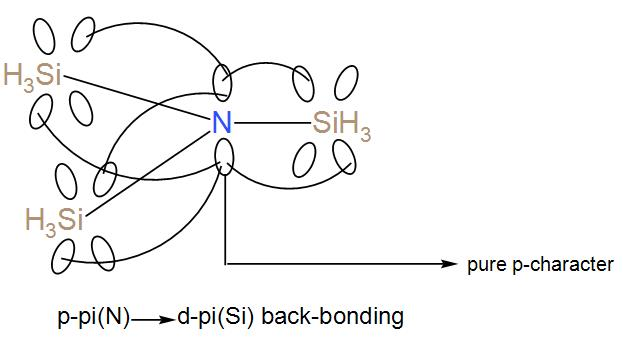
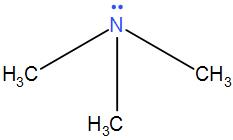
Now let’s discuss ${{\left( C{{H}_{3}} \right)}_{3}}N$. We know nitrogen has 5 electrons in its valence shell and here 3 of them are occupied with the methyl group. The other two exist as a lone pair. It means that nitrogen can undergo back-bonding but carbon does not have energetically accessible empty d-orbital to undergo back-bonding. Therefore, it has no back-bonding which will help it to gain the planar structure. Thus, it takes up tetrahedral geometry.
Also, we can see that $Si{{H}_{3}}$ is a bulky group. Even if it attained the tetrahedral structure where the bond angles are ${{109}^{\circ }}$ , it wouldn’t stay there due to repulsion of the bulky group and eventually attain the bond angle of ${{120}^{\circ }}$ thus attaining the planar structure.
We can understand from the above discussion that due to $p\pi \left( N \right)\to d\pi \left( Si \right)$ back-bonding and steric factors, ${{\left( Si{{H}_{3}} \right)}_{3}}N$ it is $s{{p}^{2}}$ hybridised and ${{\left( C{{H}_{3}} \right)}_{3}}N$ nitrogen is $s{{p}^{3}}$ hybridised.
NOTE: Although VSEPR is quite successful in determining the geometries of molecules there are certain drawbacks to this theory. This theory fails to determine the shape of the isoelectronic species. It also does not take the relative size of the constituents under consideration. It is unable to explain the atomic orbital overlaps.
Complete step by step solution:
We know that according to the VSEPR theory that is the Valence Shell Electron Pair Repulsion theory, the hybridisation of the atom can tell us about its bonding and its structure.
According to VSEPR theory, $s{{p}^{3}}$ hybridisation has a tetrahedral geometry and $s{{p}^{2}}$ hybridisation has a trigonal planar structure. Trigonal planar geometry is planar and tetrahedral geometry is not planar as one of the atoms lie above the plane.
Now in the given question, if we discuss why they take up the particular geometry, we can explain the hybridisation on its basis.
Firstly let’s discuss ${{\left( Si{{H}_{3}} \right)}_{3}}N$. We know that nitrogen has 5 electrons in the valence shell and after forming ${{\left( Si{{H}_{3}} \right)}_{3}}N$, it still has a pair of lone-pair on it. Now, these lone pairs of electrons can undergo back-bonding with the Si-atom as it has energetically accessible d-orbitals which can undergo back-bonding with the lone pair, p-orbital of nitrogen. Due to this $p\pi \left( N \right)\to d\pi \left( Si \right)$ back-bonding it attains a planar structure.

Now let’s discuss ${{\left( C{{H}_{3}} \right)}_{3}}N$. We know nitrogen has 5 electrons in its valence shell and here 3 of them are occupied with the methyl group. The other two exist as a lone pair. It means that nitrogen can undergo back-bonding but carbon does not have energetically accessible empty d-orbital to undergo back-bonding. Therefore, it has no back-bonding which will help it to gain the planar structure. Thus, it takes up tetrahedral geometry.

Also, we can see that $Si{{H}_{3}}$ is a bulky group. Even if it attained the tetrahedral structure where the bond angles are ${{109}^{\circ }}$ , it wouldn’t stay there due to repulsion of the bulky group and eventually attain the bond angle of ${{120}^{\circ }}$ thus attaining the planar structure.
We can understand from the above discussion that due to $p\pi \left( N \right)\to d\pi \left( Si \right)$ back-bonding and steric factors, ${{\left( Si{{H}_{3}} \right)}_{3}}N$ it is $s{{p}^{2}}$ hybridised and ${{\left( C{{H}_{3}} \right)}_{3}}N$ nitrogen is $s{{p}^{3}}$ hybridised.
NOTE: Although VSEPR is quite successful in determining the geometries of molecules there are certain drawbacks to this theory. This theory fails to determine the shape of the isoelectronic species. It also does not take the relative size of the constituents under consideration. It is unable to explain the atomic orbital overlaps.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




