
In $I{F_3}$ the bond angle of $F - I - F$ is
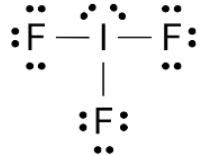
(A) Equal to ${90^ \circ }$
(B) Less that ${90^ \circ }$
(C) Greater than ${90^ \circ }$
(D) None of the above
Answer
233.1k+ views
Hint: In this question we have to justify the angle between $F - I - F$ whose chemical formula is $I{F_3}$ . As for the solution of these types of questions we have to know the geometry, hybridization and structure of the molecule which is given in the question. By which we can easily find the angle which is made between the elements.
Complete step by step solution:
As by using VSEPR theory in this question we can easily find the geometry of the given molecule.
By using the identity firstly, we justify the hybridization of the molecule,
The hybridization of $I{F_3}$ is $s{p_3}d$ . As by using the hybridization we can also justify the structure and geometry of the molecule.
The geometry of the IF3 molecule is Trigonal bipyramidal.
For the first time, X-ray analysis has been used to determine the T-shaped molecular structure of unstable IF3. Due to two weak connections connecting through nearby molecules' fluorine atoms, the iodine atom is pentagonal-planar coordinated in the solid state.
By using the geometry, we can also justify the bond angle of the molecule,
As per which the lone pair is also present on the central atom, we calculated the bond angle is \[{88.5^ \circ }\]in the $F - I - F$ .
Therefore, the correct answer is Less than ${90^ \circ }$ .
Hence, the correct option is (B)
Note: Based on the number of valence shell electron bond pairs between the atoms in a molecule or ion, the valence shell electron pair repulsion (VSEPR) theory is a model used to predict 3-D molecular shape. According to this hypothesis, electron pairs will position themselves to reduce the consequences of their mutual repulsion.
Complete step by step solution:
As by using VSEPR theory in this question we can easily find the geometry of the given molecule.
By using the identity firstly, we justify the hybridization of the molecule,
The hybridization of $I{F_3}$ is $s{p_3}d$ . As by using the hybridization we can also justify the structure and geometry of the molecule.
The geometry of the IF3 molecule is Trigonal bipyramidal.
For the first time, X-ray analysis has been used to determine the T-shaped molecular structure of unstable IF3. Due to two weak connections connecting through nearby molecules' fluorine atoms, the iodine atom is pentagonal-planar coordinated in the solid state.
By using the geometry, we can also justify the bond angle of the molecule,
As per which the lone pair is also present on the central atom, we calculated the bond angle is \[{88.5^ \circ }\]in the $F - I - F$ .
Therefore, the correct answer is Less than ${90^ \circ }$ .
Hence, the correct option is (B)
Note: Based on the number of valence shell electron bond pairs between the atoms in a molecule or ion, the valence shell electron pair repulsion (VSEPR) theory is a model used to predict 3-D molecular shape. According to this hypothesis, electron pairs will position themselves to reduce the consequences of their mutual repulsion.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




