
In graphite, electrons are:
(A) Localized on each carbon atom
(B) Spread out between the sheets
(C) localized on every third carbon atom
(D) present in antibonding orbital
Answer
239.4k+ views
Hint: Graphite is an allotrope of carbon element. When the same element is present in more than one form they are called allotropes.
Carbon is present in three forms in nature i.e. Diamond, Graphite and fullerene.
Complete step by step answer:
Carbon atom in graphite present in$s{p^2}$ hybridized state.
Electronic configuration of C is$ - 1{s^2}2{s^2}2p{x^1}2p{y^1}$
At ground state =
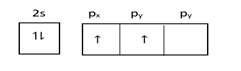
At excited state =
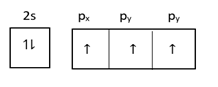
In graphite $1s$ and $2p$ orbital hybridize and form $s{p^2}$ hybridized orbital and one orbital remains hybridized.
Hybridized state=
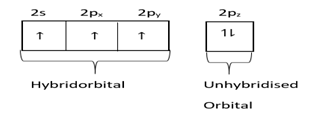
Three hybrid orbits contain one electron each.
These electrons form a covalent bond with the hybrid orbital of other C-atoms.
Each carbon atom forms a covalent bond with another carbon atom. They form interlinked six membered rings of carbon atoms. The remaining half filled unhybridized $2{p^2}$orbital is used for$\pi $bonding. So that layers of carbon atoms i.e. graphite is formed.
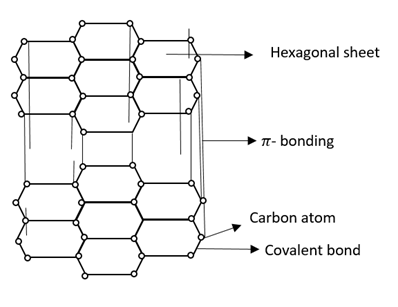
Graphite has delocalized molecular orbital and delocalized electrons and is free to move in delocalized molecular orbital.
Therefore, electrons get spread between the sheets.
Therefore, from the above explanation, the correct option is B
Additional information:
Graphite is a good conductor of heat and electricity. The layer of carbon atoms in graphite can slide over another layer. So it is used in lubricants for reducing friction.
Note: Graphite forms hexagonal layers and these layers of carbon atom are held together by weak Van der Waals forces of attraction.
Carbon atoms have a unique property to link with itself called catenation. Due to this property allotropes of carbon form giant molecules.
Carbon is present in three forms in nature i.e. Diamond, Graphite and fullerene.
Complete step by step answer:
Carbon atom in graphite present in$s{p^2}$ hybridized state.
Electronic configuration of C is$ - 1{s^2}2{s^2}2p{x^1}2p{y^1}$
At ground state =

At excited state =

In graphite $1s$ and $2p$ orbital hybridize and form $s{p^2}$ hybridized orbital and one orbital remains hybridized.
Hybridized state=

Three hybrid orbits contain one electron each.
These electrons form a covalent bond with the hybrid orbital of other C-atoms.
Each carbon atom forms a covalent bond with another carbon atom. They form interlinked six membered rings of carbon atoms. The remaining half filled unhybridized $2{p^2}$orbital is used for$\pi $bonding. So that layers of carbon atoms i.e. graphite is formed.

Graphite has delocalized molecular orbital and delocalized electrons and is free to move in delocalized molecular orbital.
Therefore, electrons get spread between the sheets.
Therefore, from the above explanation, the correct option is B
Additional information:
Graphite is a good conductor of heat and electricity. The layer of carbon atoms in graphite can slide over another layer. So it is used in lubricants for reducing friction.
Note: Graphite forms hexagonal layers and these layers of carbon atom are held together by weak Van der Waals forces of attraction.
Carbon atoms have a unique property to link with itself called catenation. Due to this property allotropes of carbon form giant molecules.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE Main Mock Test 2025-26: Principles Related To Practical

JEE Main 2025-26 Organic Compounds Containing Nitrogen Mock Test

JEE Main 2025-26 Mock Test: Organic Compounds Containing Oxygen

JEE Main 2025-26 Redox Reactions & Electro Mock Test

JEE Main Solutions Mock Test 1-2 (2025-26): Free Practice & Answers

Trending doubts
JEE Main 2026: Session 1 Results Out and Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

JEE Main Participating Colleges 2026 - A Complete List of Top Colleges

Understanding Electromagnetic Waves and Their Importance

Common Ion Effect: Concept, Applications, and Problem-Solving

Hybridisation in Chemistry – Concept, Types & Applications

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

JEE Advanced 2026 - Exam Date (Released), Syllabus, Registration, Eligibility, Preparation, and More

NCERT Solutions For Class 12 Chemistry Chapter 2 Electrochemistry - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The D And F Block Elements - 2025-26

CBSE Notes Class 12 Chemistry Chapter 1 - Solutions - 2025-26




